Modern Atomic Theory Rutherfords Model of the Atom





- Slides: 21

Modern Atomic Theory

Rutherford’s Model of the Atom • Nucleus composed of protons (positively charged particles) and neutrons (neutral particles). • Nucleus is very small compared to the rest of the atom. • Thought electrons might revolve around the nucleus • PROBLEM: – Why doesn’t the atom collapse? – Shouldn’t the negatively charged electrons and positively charged protons of the nucleus attract each other?

Wave-Particle Duality • To understand better about the atom, it was necessary to study light and how it transmits energy • Max Plank – Light (a wave) has properties of a particle • De Broglie – Particles can have wave-like properties Wave-Particle Duality states: • Light consists of BOTH waves and particles

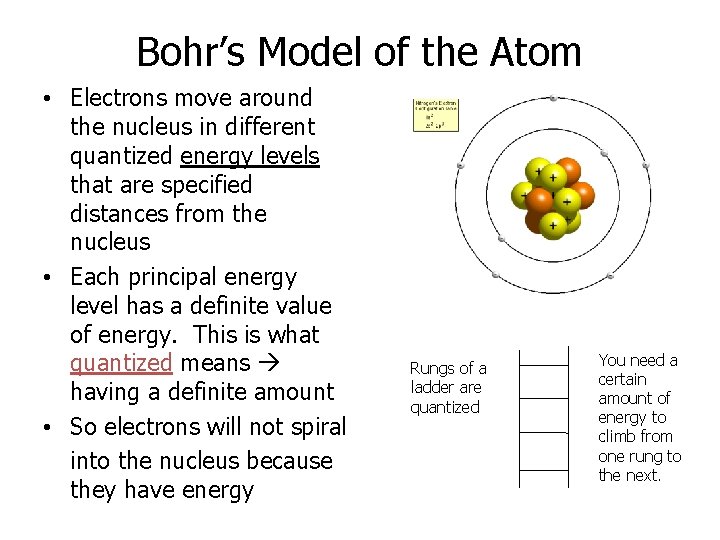
Bohr’s Model of the Atom • Electrons move around the nucleus in different quantized energy levels that are specified distances from the nucleus • Each principal energy level has a definite value of energy. This is what quantized means having a definite amount • So electrons will not spiral into the nucleus because they have energy Rungs of a ladder are quantized You need a certain amount of energy to climb from one rung to the next.

Wave Mechanics Model of the Atom -currently accepted model (aka orbital model) -Electrons may be found in probable locations called “electron clouds”, or orbitals -electrons do not move in set orbits but move randomly within electron clouds (orbitals) which are located inside the atom but outside the nucleus -it is impossible to know the exact location of an electron at any moment.

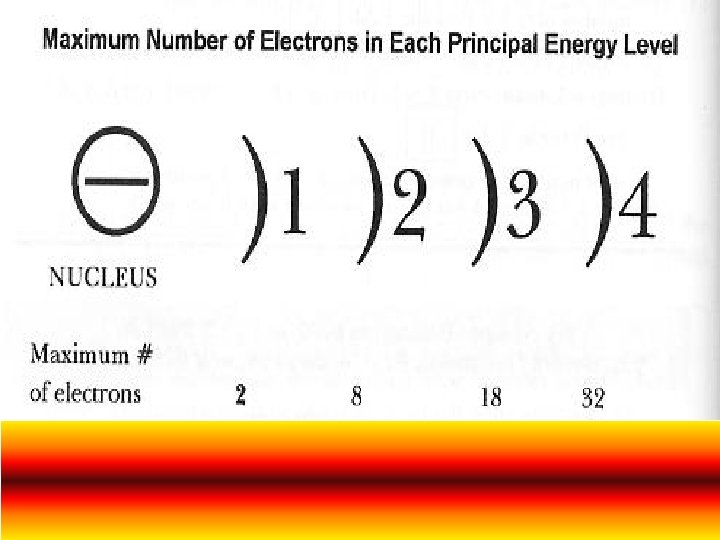
Principal Energy Level (shells or principal quantum numbers) Principal energy levels show far the electron is from the nucleus. The first energy level is closest to the nucleus, while other energy levels are further away from the nucleus. Electrons in the first energy level have the lowest energy. Those in the second energy level have more energy than those in the first and so on First Principal Energy Level can only hold 2 electrons Second Principal Energy Level can only hold 8 electrons Third Principal Energy Level can only hold 18 electrons Fourth Principal Energy Level can only hold 32 electrons


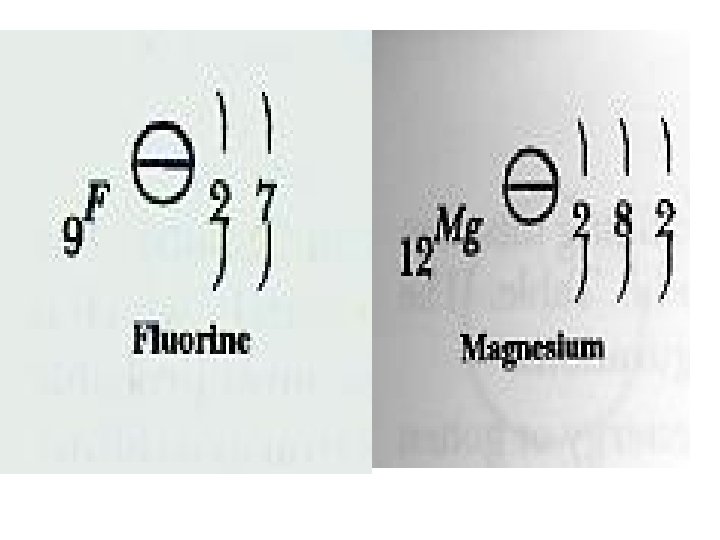
Electron Configuration shows how many electrons are in each principal energy level. Example 1: Fluorine has an atomic number of 9, therefore Fluorine has 9 protons and since it has no charge, it has 9 electrons. 1. You put 2 electrons in principal energy level 1. Principal energy level 1 can only hold 2 electrons 2. The next 7 electrons are in principal energy level 2. Example 2: Magnesium has an atomic number of 12, therefore it has 12 electrons. 1. You put 2 electrons in principal energy level 1. Principal energy level 1 can only hold 2 electrons 2. The next 8 electrons are in principal energy level 2. Principal energy level 2 can only hold 8 electrons 3. The next 2 electrons are in principal energy level 3.


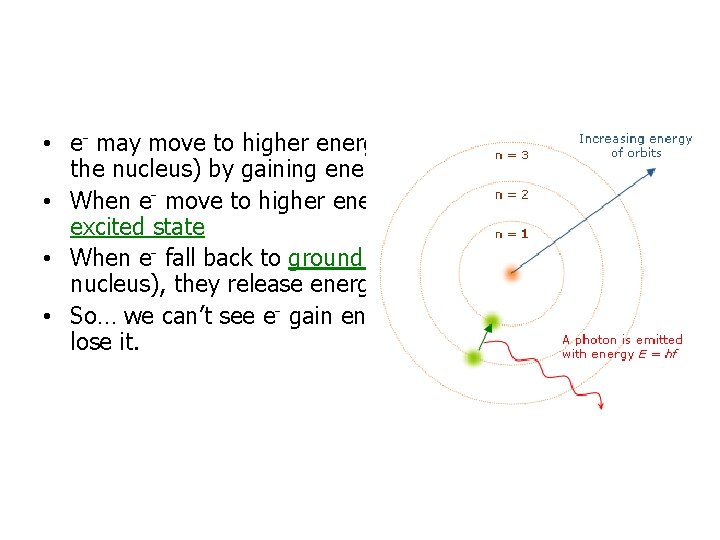
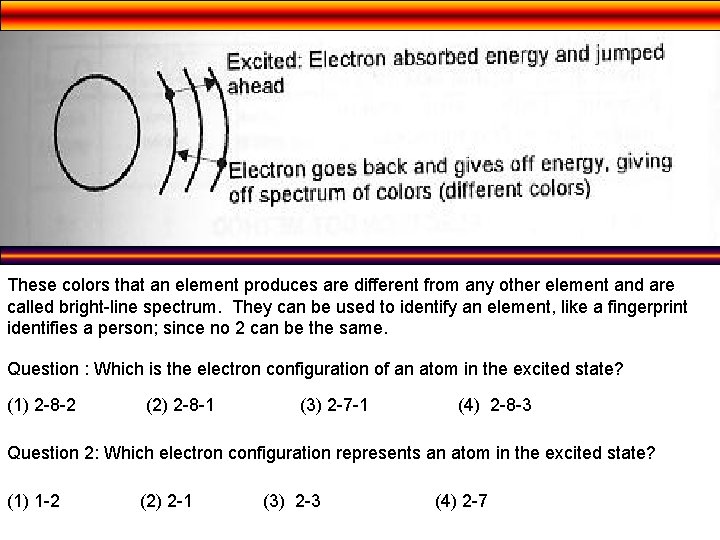
• e- may move to higher energy levels (further away from the nucleus) by gaining energy • When e- move to higher energy levels they are in an excited state • When e- fall back to ground state (closest to the nucleus), they release energy in the form of visible light • So… we can’t see e- gain energy, we can only see them lose it.

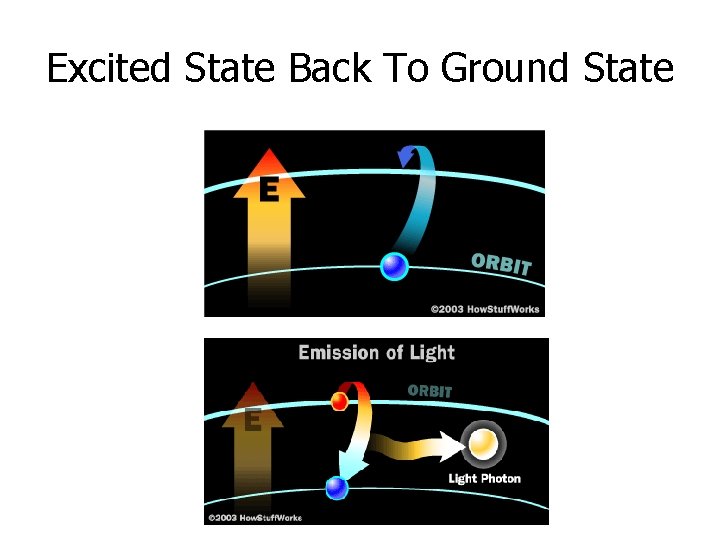
Excited State Back To Ground State

Ground State Electron Configuration: -the most stable state of the element -arrangement found in nature -arrangement given in periodic table Excited State Configuration: -any arrangement of electrons, other than what is given in the periodic table -occurs when an atom absorbs energy and the electron moves away from the nucleus (electrons jump ahead to a higher energy level, leaving one of the inner levels partly empty) -very unstable state, very short life time -when the electron goes back towards the nucleus, energy is released as spectra Emission Spectra -when an electron moves from a higher energy level (farther from the nucleus) to a lower energy level (closer to the nucleus), energy is released and spectra are emitted. This energy is emitted in a form of light. The energy (color) of the light depends on how far the electron falls.

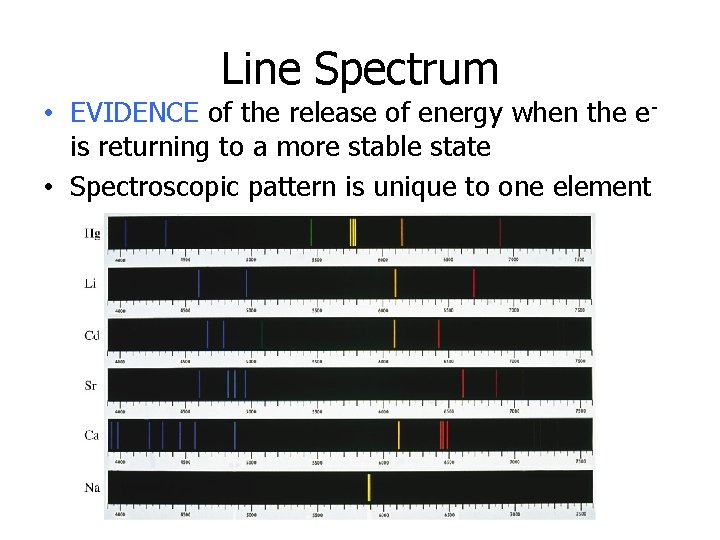
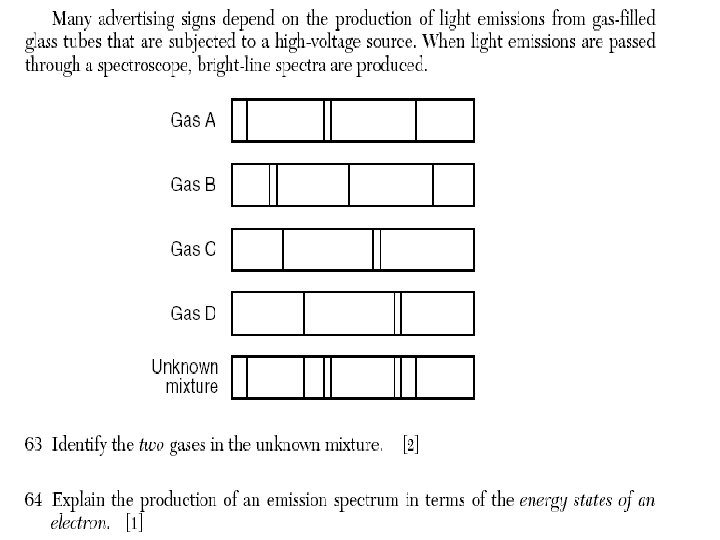
Line Spectrum • EVIDENCE of the release of energy when the eis returning to a more stable state • Spectroscopic pattern is unique to one element

These colors that an element produces are different from any other element and are called bright-line spectrum. They can be used to identify an element, like a fingerprint identifies a person; since no 2 can be the same. Question : Which is the electron configuration of an atom in the excited state? (1) 2 -8 -2 (2) 2 -8 -1 (3) 2 -7 -1 (4) 2 -8 -3 Question 2: Which electron configuration represents an atom in the excited state? (1) 1 -2 (2) 2 -1 (3) 2 -3 (4) 2 -7


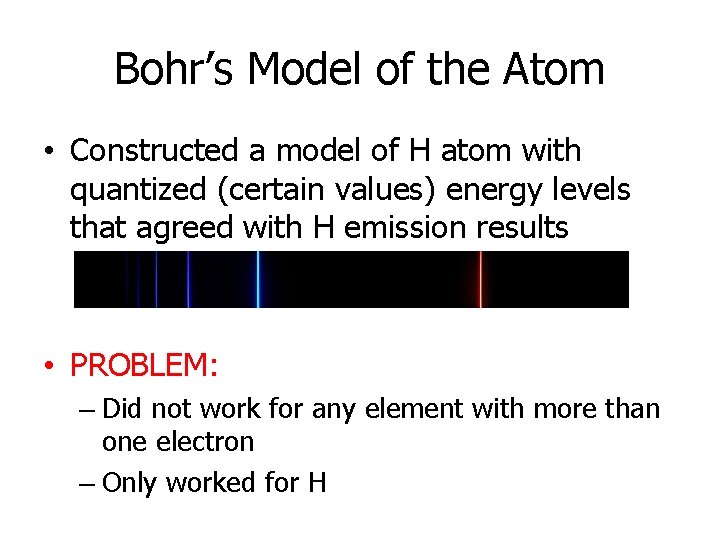
Bohr’s Model of the Atom • Constructed a model of H atom with quantized (certain values) energy levels that agreed with H emission results • PROBLEM: – Did not work for any element with more than one electron – Only worked for H

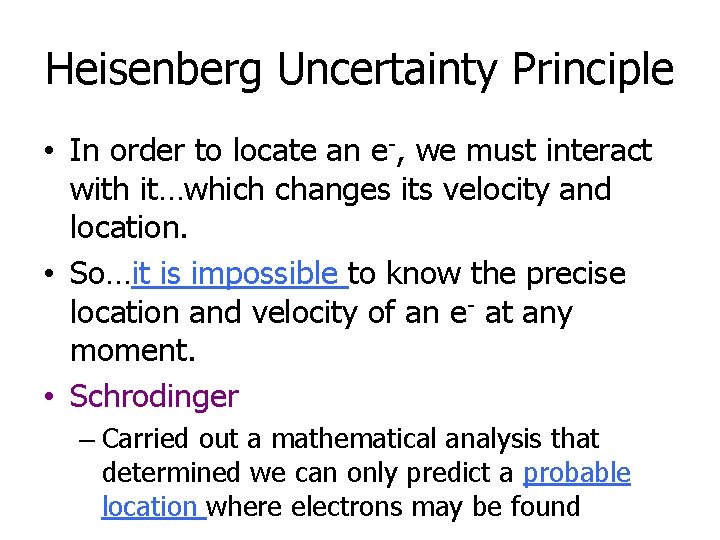
Heisenberg Uncertainty Principle • In order to locate an e-, we must interact with it…which changes its velocity and location. • So…it is impossible to know the precise location and velocity of an e- at any moment. • Schrodinger – Carried out a mathematical analysis that determined we can only predict a probable location where electrons may be found

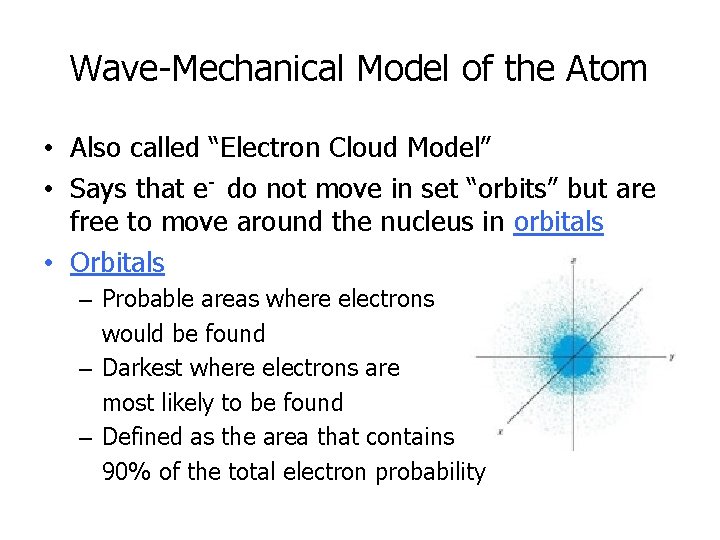
Wave-Mechanical Model of the Atom • Also called “Electron Cloud Model” • Says that e- do not move in set “orbits” but are free to move around the nucleus in orbitals • Orbitals – Probable areas where electrons would be found – Darkest where electrons are most likely to be found – Defined as the area that contains 90% of the total electron probability


Valence Electrons – Electrons from the outermost (highest) energy level. These are the electrons that participate in bonding Kernel – Represents the nucleus of the element and all electrons except valence electrons Lewis Electron Dot Structure – The symbol of the element is surrounded by valence electrons.

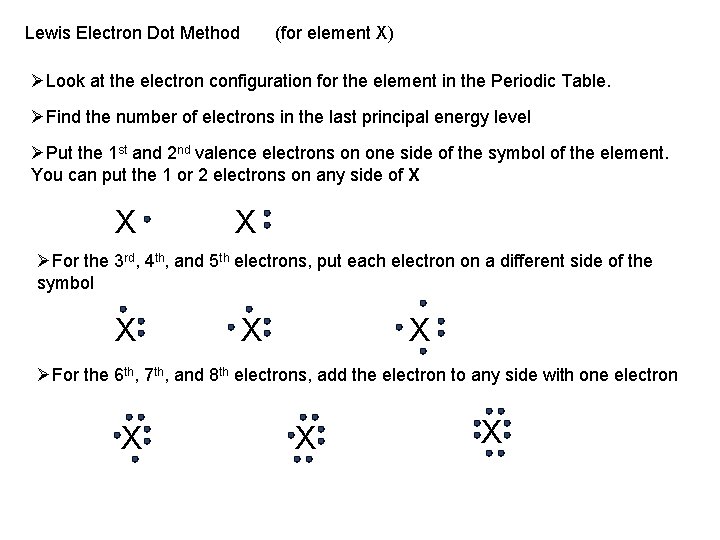
Lewis Electron Dot Method (for element X) ØLook at the electron configuration for the element in the Periodic Table. ØFind the number of electrons in the last principal energy level ØPut the 1 st and 2 nd valence electrons on one side of the symbol of the element. You can put the 1 or 2 electrons on any side of X X X ØFor the 3 rd, 4 th, and 5 th electrons, put each electron on a different side of the symbol X X X ØFor the 6 th, 7 th, and 8 th electrons, add the electron to any side with one electron X X X