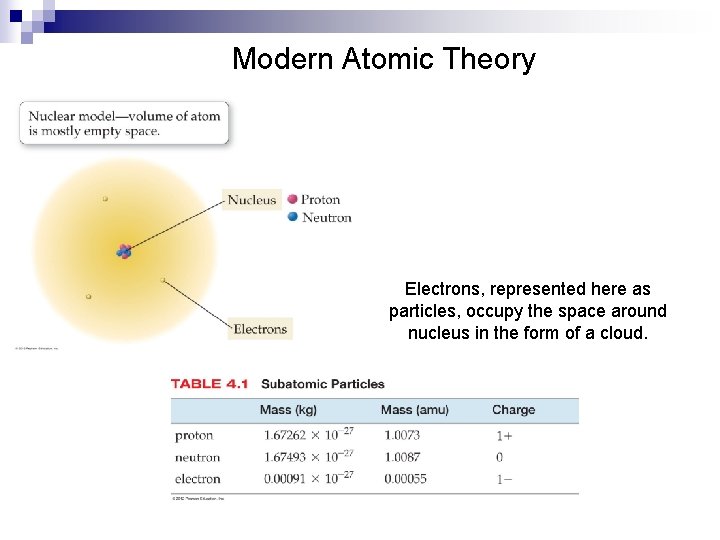
Modern Atomic Theory Electrons represented here as particles


- Slides: 6

Modern Atomic Theory Electrons, represented here as particles, occupy the space around nucleus in the form of a cloud.

It is the number of protons in the nucleus that determines the identity of an atom. The atomic number (Z) of an atom is equal to the number of protons. This number uniquely identifies an element. The atomic number is the whole number located at the element’s square on the periodic table. It is the number of protons in the nucleus that determines the identity of an element.

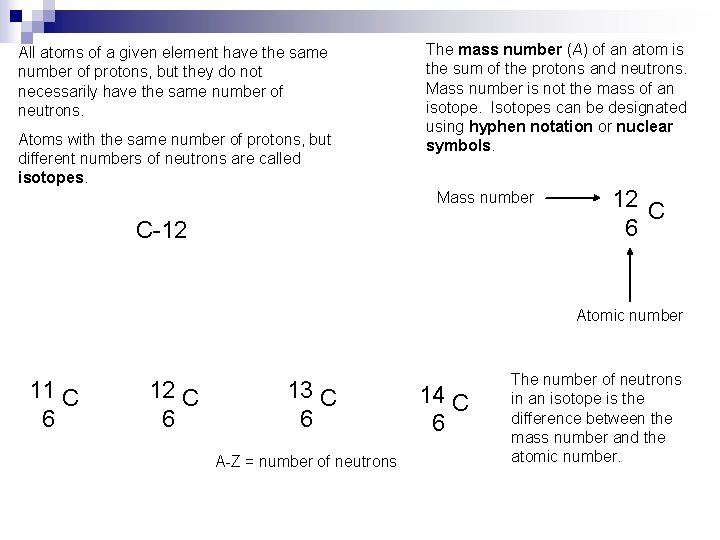
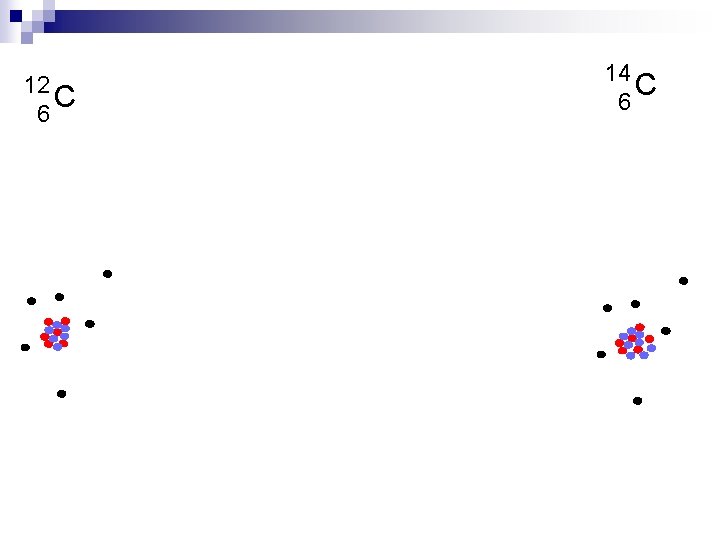
All atoms of a given element have the same number of protons, but they do not necessarily have the same number of neutrons. Atoms with the same number of protons, but different numbers of neutrons are called isotopes. The mass number (A) of an atom is the sum of the protons and neutrons. Mass number is not the mass of an isotope. Isotopes can be designated using hyphen notation or nuclear symbols. Mass number C-12 12 C 6 Atomic number 11 C 6 12 C 6 13 C 6 A-Z = number of neutrons 14 C 6 The number of neutrons in an isotope is the difference between the mass number and the atomic number.

12 C 6 14 C 6

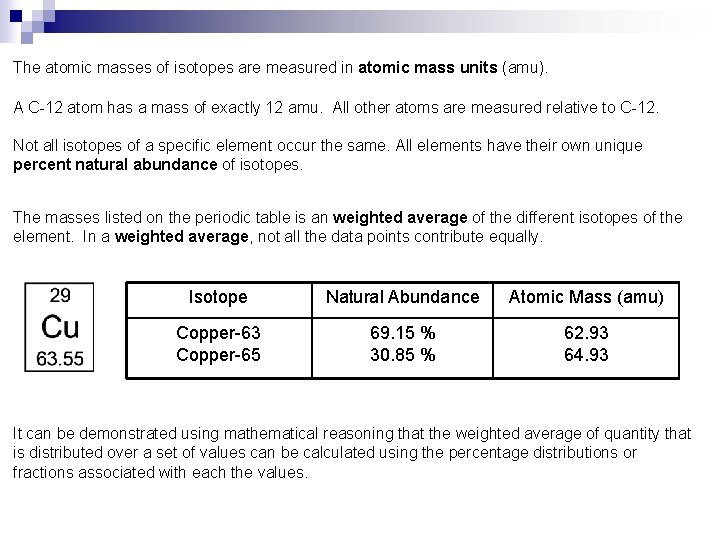
The atomic masses of isotopes are measured in atomic mass units (amu). A C-12 atom has a mass of exactly 12 amu. All other atoms are measured relative to C-12. Not all isotopes of a specific element occur the same. All elements have their own unique percent natural abundance of isotopes. The masses listed on the periodic table is an weighted average of the different isotopes of the element. In a weighted average, not all the data points contribute equally. Isotope Natural Abundance Atomic Mass (amu) Copper-63 Copper-65 69. 15 % 30. 85 % 62. 93 64. 93 It can be demonstrated using mathematical reasoning that the weighted average of quantity that is distributed over a set of values can be calculated using the percentage distributions or fractions associated with each the values.

The concept of average atomic mass can be summarized as follows: The atomic masses listed on the periodic table are averages that are weighted according to the abundances of the naturally occurring isotopes. Since the abundances can differ greatly from each other, the average atomic masses are weighted towards the more abundant isotopes. Furthermore, the mass number of the most abundant isotope will usually be close to the average atomic mass rounded to the nearest whole number. Most situations do not involve isotopically pure samples of matter. Instead, they involve samples that contain a mixture of all the isotopes, with a distribution of the isotopes corresponding to the natural abundances. By using the average atomic masses of the atoms, instead of the masses of individual isotopes, the distributions of the different isotopes can be worked into calculations that involve the masses of large numbers of atoms and particles. Only in situations that involve individual particles or isotopically pure samples of matter are the masses of individual isotopes needed in calculations.