Modern Atomic Model Electron modeling To understand electrons












- Slides: 31

Modern Atomic Model

Electron modeling… ¥To understand electrons, scientists began comparing them to something they knew - light.

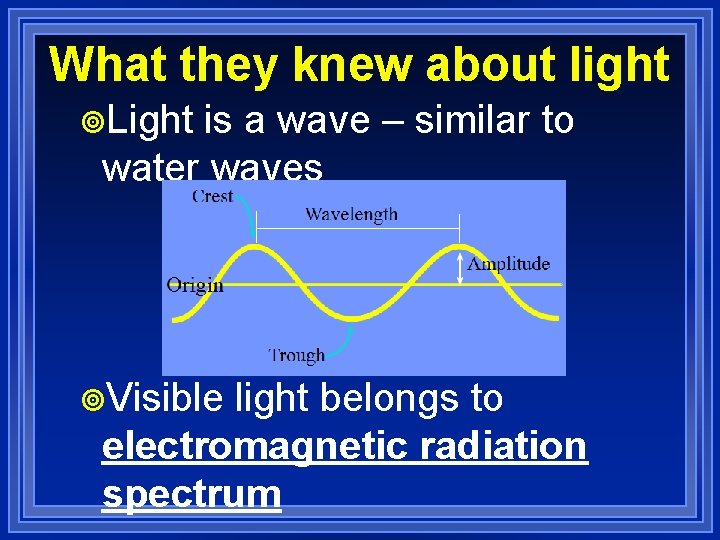
What they knew about light ¥Light is a wave – similar to water waves ¥Visible light belongs to electromagnetic radiation spectrum

Behavior of light ¥All forms of EMR have common properties: ¥Amplitude ¥Wavelength ¥Frequency ¥Speed

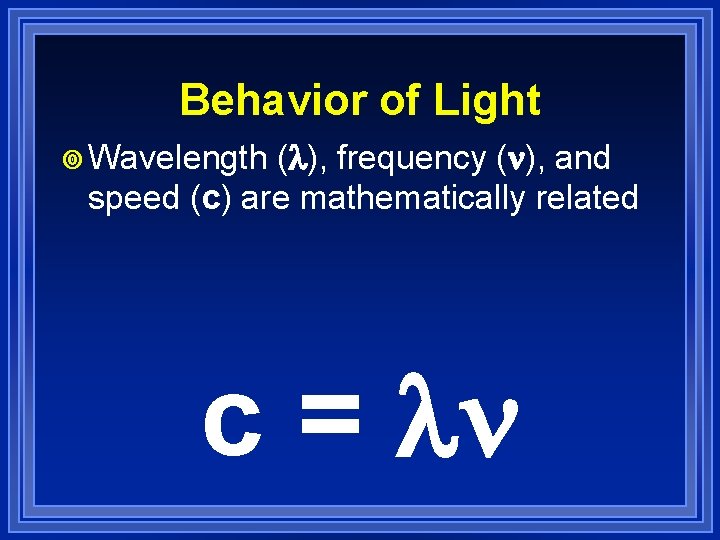
Behavior of Light ¥ Wavelength (l), frequency ( ), and speed (c) are mathematically related c = l

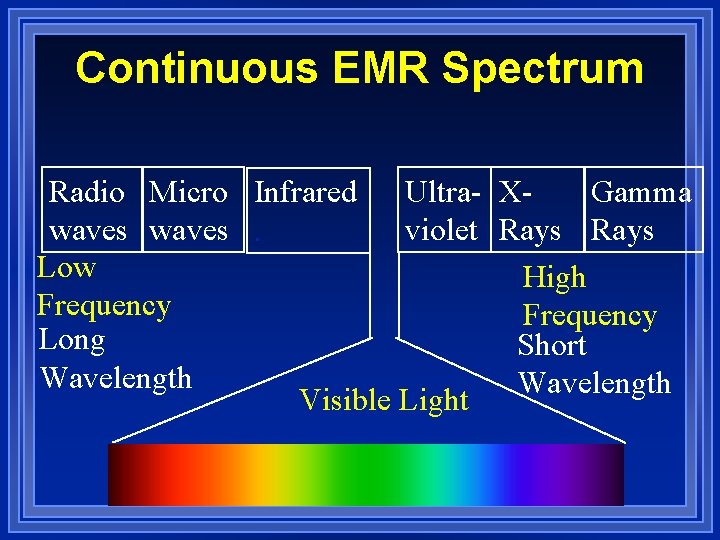
Behavior of light Frequency and wavelength: ¥ Are inversely related ¥As one goes up, the other goes down. ¥ Different frequencies of light go with different colors of light. ¥ There is a wide variety of frequencies ¥The whole range is called a continuous spectrum

Continuous EMR Spectrum Radio Micro Infrared Ultra- XGamma waves. violet Rays Low High Frequency Long Short Wavelength Visible Light

Wave model had problems At the start of the 20 th century, scientists made observations of light that didn’t fit the model…

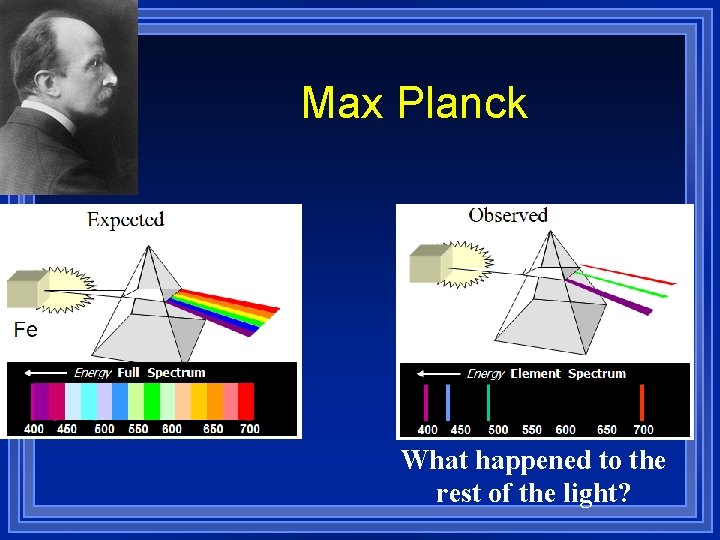
Wave model problems Black body radiation ¥ In 1900, Max Planck heated matter that didn’t burn and studied the radiation emitted. It was predicted that matter could absorb or emit any quantity of energy. His experimental data did not fit that statement.

Max Planck What happened to the rest of the light?

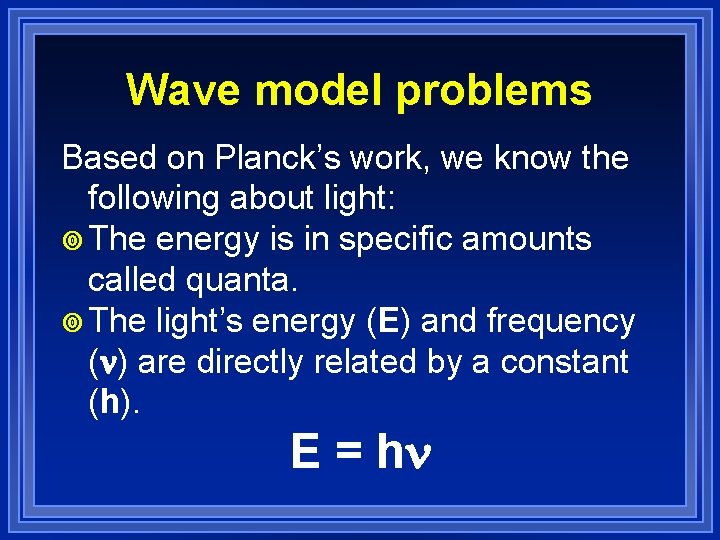
Wave model problems Based on Planck’s work, we know the following about light: ¥ The energy is in specific amounts called quanta. ¥ The light’s energy (E) and frequency ( ) are directly related by a constant (h). E = h

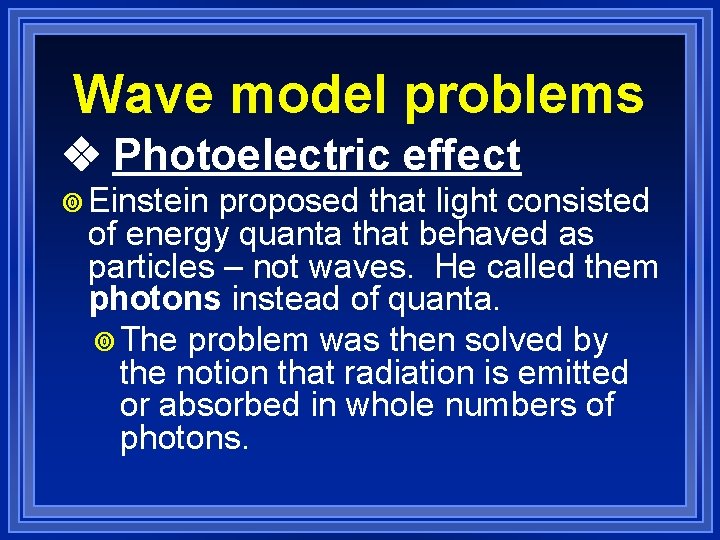
Wave model problems ¥Planck’s ideas weren’t accepted until some time later when Albert Einstein used Planck’s equation to work on solving the photoelectric effect.

Wave model problems Photoelectric effect ¥Light shining on certain metals can eject electrons. ¥Simulation

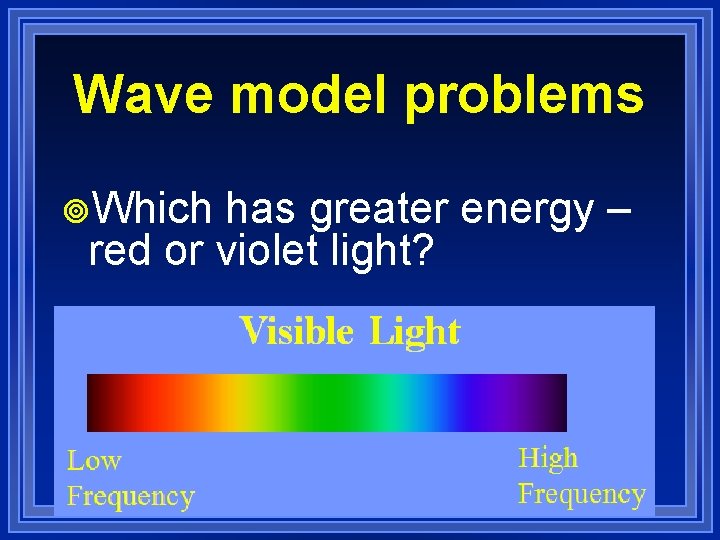
Wave model problems ¥Which has greater energy – red or violet light?

Wave model problems Photoelectric effect ¥ Einstein proposed that light consisted of energy quanta that behaved as particles – not waves. He called them photons instead of quanta. ¥ The problem was then solved by the notion that radiation is emitted or absorbed in whole numbers of photons.

Wave model problems Photoelectric effect ¥It was later proven that light could definitely act as a particle. So, we now have light acting as both a wave and a particle.

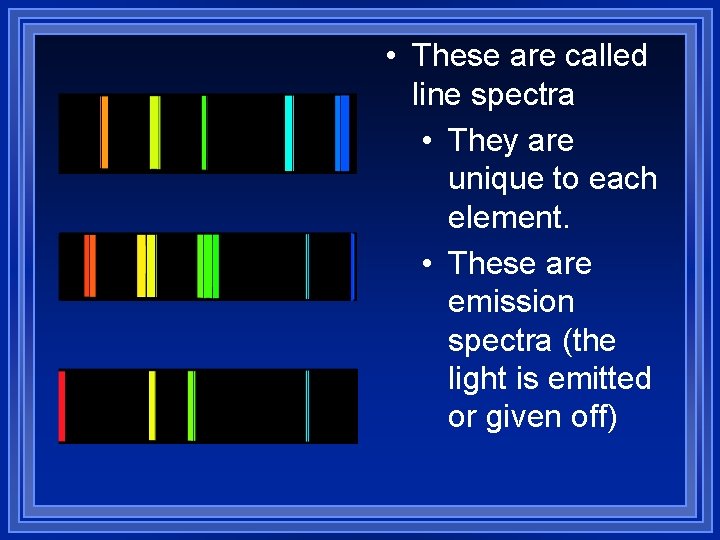
Wave model problems Bright line spectrum ¥Scientists noticed that you could vaporize an element in a flame to produce different flame colors. You can then use a prism to sort the colors to produce a line spectrum (only certain colors are produced).

Prism with white light ¥ White light is made up of all the colors of the visible spectrum. ¥ Passing it through a prism separates it.

If the light entering the prism is not white… ¥ By adding energy to a gas, we can get the gas to give off colored light ¥ Passing this light through a prism does something different than white light

Wave model problems Bright line spectrum ¥Problem: Each element produced a different line spectrum.

• These are called line spectra • They are unique to each element. • These are emission spectra (the light is emitted or given off)

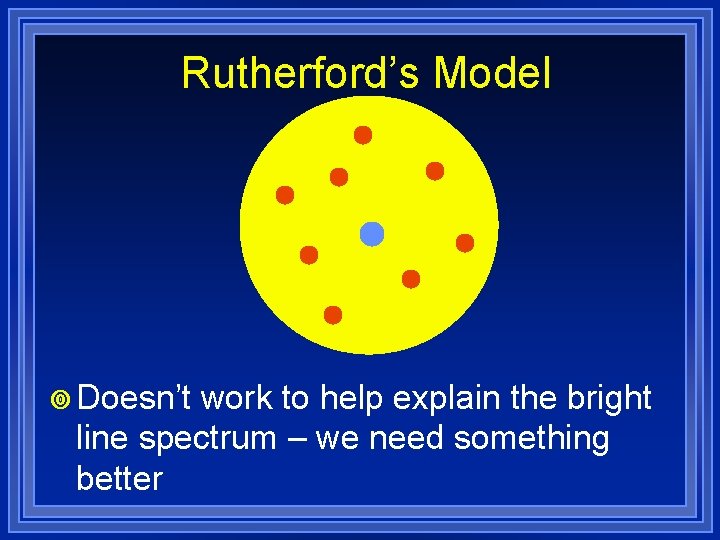
Rutherford’s Model ¥ Doesn’t work to help explain the bright line spectrum – we need something better

Bohr’s Model: An explanation for the observed atomic spectra

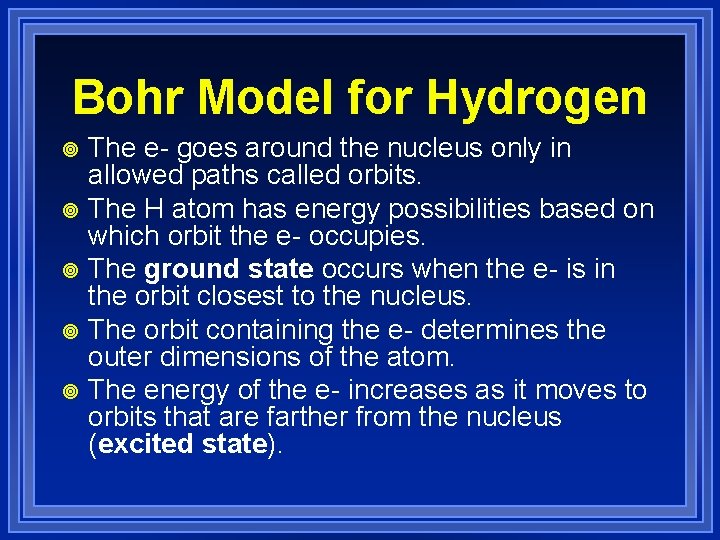
Bohr Model for Hydrogen The e- goes around the nucleus only in allowed paths called orbits. ¥ The H atom has energy possibilities based on which orbit the e- occupies. ¥ The ground state occurs when the e- is in the orbit closest to the nucleus. ¥ The orbit containing the e- determines the outer dimensions of the atom. ¥ The energy of the e- increases as it moves to orbits that are farther from the nucleus (excited state). ¥

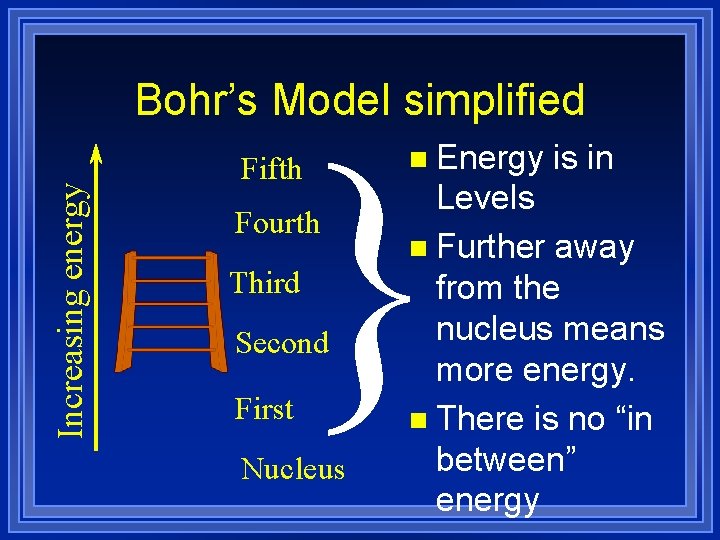
Increasing energy Bohr’s Model simplified } Fifth Fourth Third Second First Nucleus n Energy is in Levels n Further away from the nucleus means more energy. n There is no “in between” energy

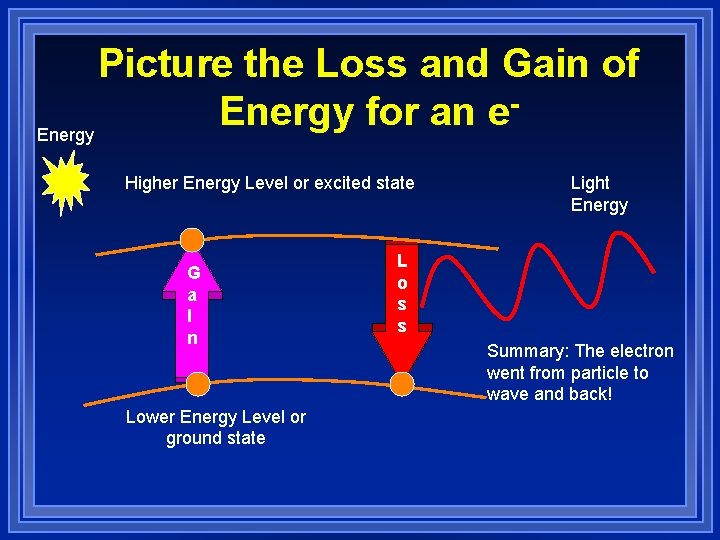
Picture the Loss and Gain of Energy for an e Energy Higher Energy Level or excited state G a I n Lower Energy Level or ground state Light Energy L o s s Summary: The electron went from particle to wave and back!

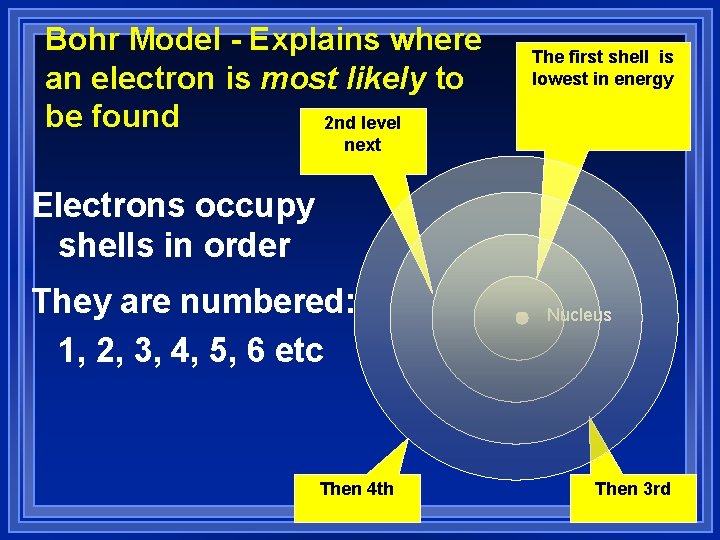
Bohr Model - Explains where an electron is most likely to be found 2 nd level The first shell is lowest in energy next Electrons occupy shells in order They are numbered: 1, 2, 3, 4, 5, 6 etc Then 4 th Nucleus Then 3 rd

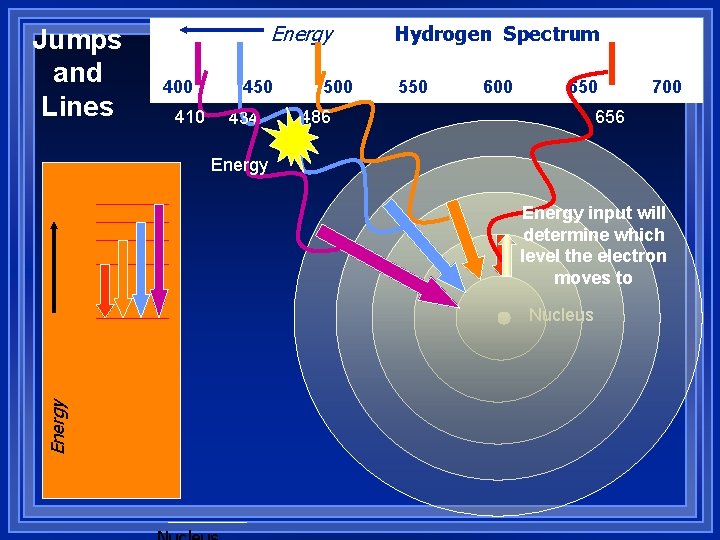
Jumps and Lines Energy 400 410 450 434 500 Hydrogen Spectrum 550 600 650 486 700 656 Energy input will determine which level the electron moves to Energy Nucleus

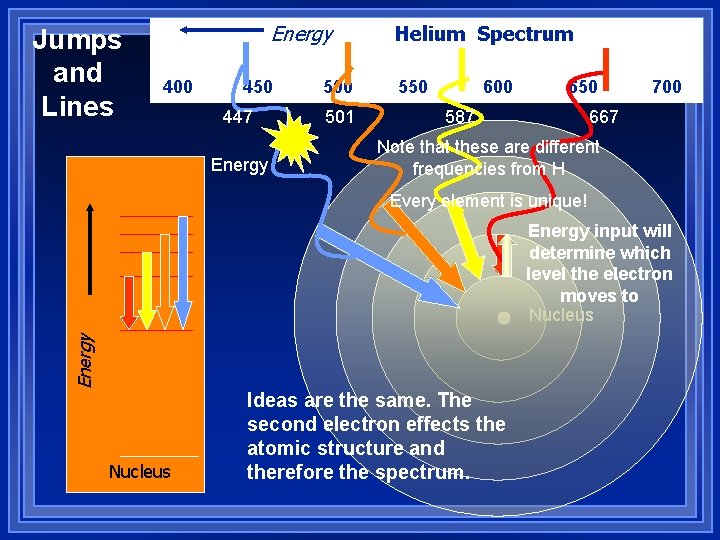
Jumps and Lines Energy 400 450 447 Energy 500 501 Helium Spectrum 550 600 650 587 700 667 Note that these are different frequencies from H Every element is unique! Energy input will determine which level the electron moves to Nucleus Ideas are the same. The second electron effects the atomic structure and therefore the spectrum.

Bohr Model Problems ¥Unfortunately, Bohr’s model only worked for H. ¥So what about the 100+ other elements?

De Broglie • Determined that particles of matter could act as waves. • Described the wavelength of moving particles. Conclusion: Matter exhibits both wave and particle properties!