Models of the Atom Model vs Theory A




- Slides: 21

Models of the Atom

Model vs. Theory • A Model is a human construct to help us better understand real world systems. • A Theory is an explanation of the natural world that can incorporate laws, hypotheses and facts. – It is testable, and can be refined or rejected. A model is used to explain a theory

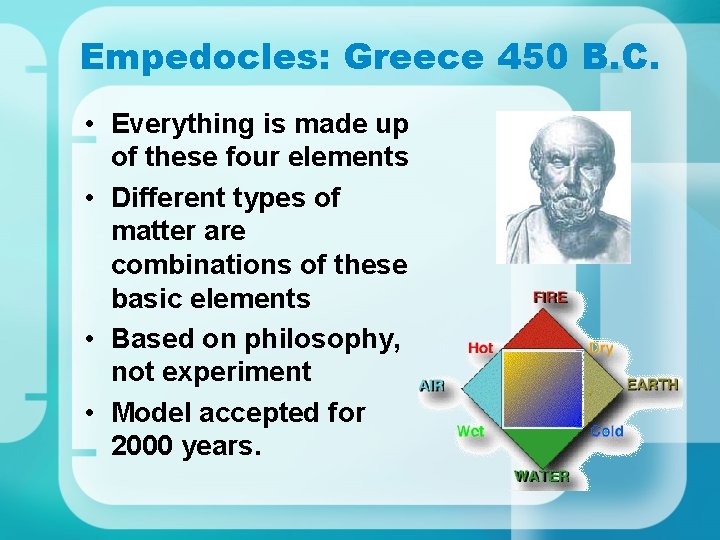
Empedocles: Greece 450 B. C. • Everything is made up of these four elements • Different types of matter are combinations of these basic elements • Based on philosophy, not experiment • Model accepted for 2000 years.

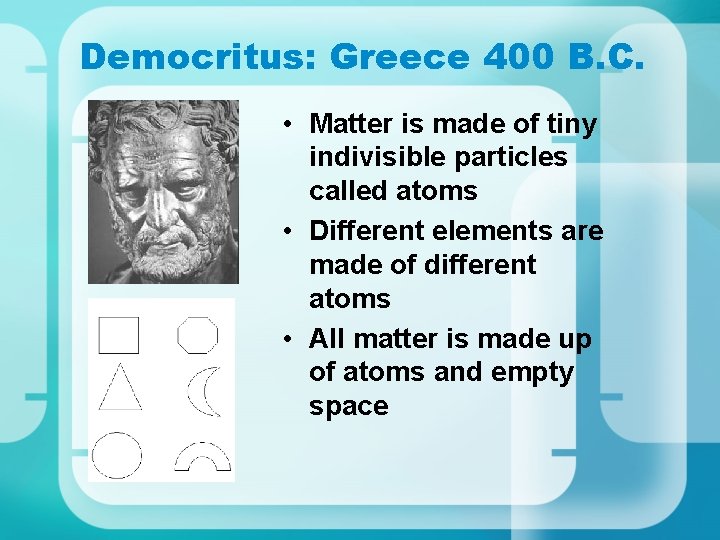
Democritus: Greece 400 B. C. • Matter is made of tiny indivisible particles called atoms • Different elements are made of different atoms • All matter is made up of atoms and empty space

Dalton: England 1650 • All matter is made of atoms too small to see. • Dalton is the reason we now use PIASM • Each element has its own kind of atom with characteristic properties • Compounds created when atoms of different elements link to form molecules • Atoms cannot be created or destroyed in a chemical reaction Billiard Ball Atom

J. J. Thomson: England 1904 • Discovered evidence of particles within the atom • His “claim to fame” was determining that atoms contain negatively charged particles called electrons • Thought that electrons were embedded in a positive sphere so that the resulting atoms are neutral or uncharged Raisin Bun Model

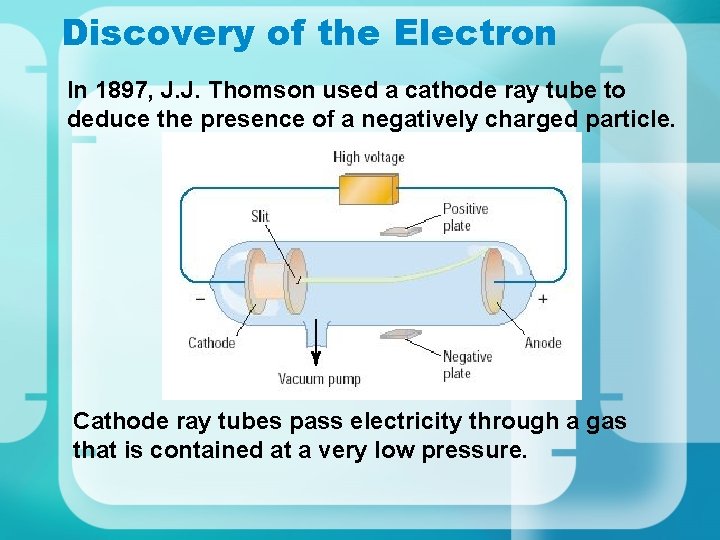
Discovery of the Electron In 1897, J. J. Thomson used a cathode ray tube to deduce the presence of a negatively charged particle. Cathode ray tubes pass electricity through a gas that is contained at a very low pressure.

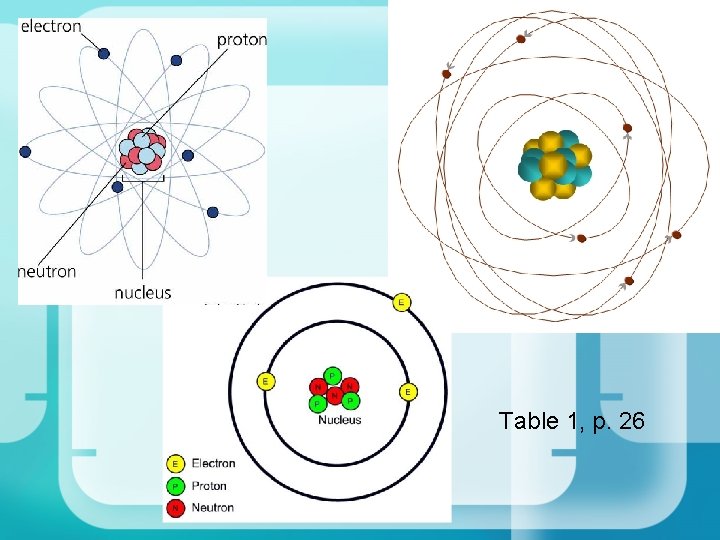
Rutherford: New Zealand 1911 • Positive centre called the nucleus • Protons are positively charged particles in the nucleus • Surrounded by mostly empty space containing rapidly moving negative electrons – At 5’ 9”, my nearest e- would be 10 km away!

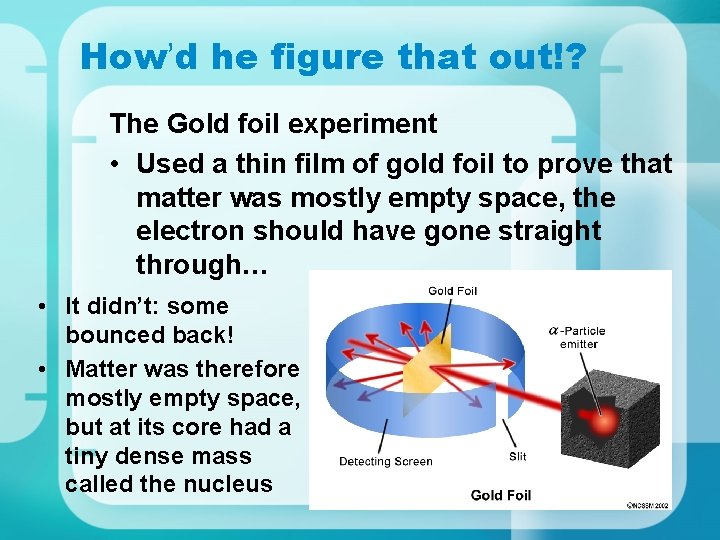
How’d he figure that out!? The Gold foil experiment • Used a thin film of gold foil to prove that matter was mostly empty space, the electron should have gone straight through… • It didn’t: some bounced back! • Matter was therefore mostly empty space, but at its core had a tiny dense mass called the nucleus

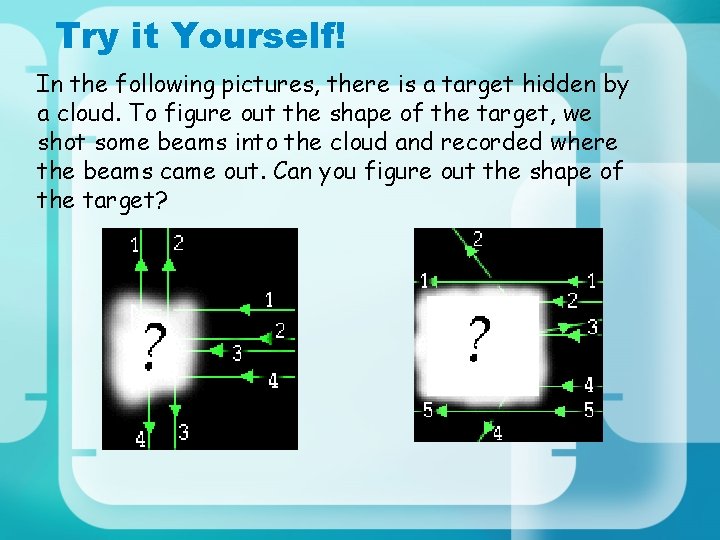
Try it Yourself! In the following pictures, there is a target hidden by a cloud. To figure out the shape of the target, we shot some beams into the cloud and recorded where the beams came out. Can you figure out the shape of the target?

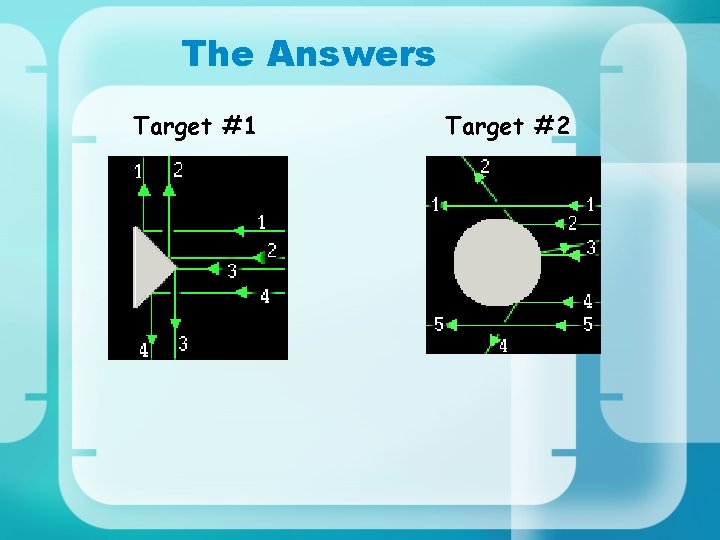
The Answers Target #1 Target #2

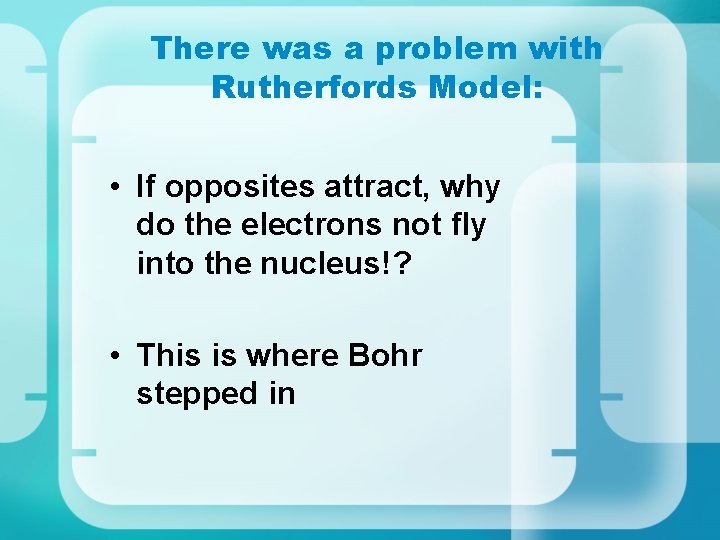
There was a problem with Rutherfords Model: • If opposites attract, why do the electrons not fly into the nucleus!? • This is where Bohr stepped in

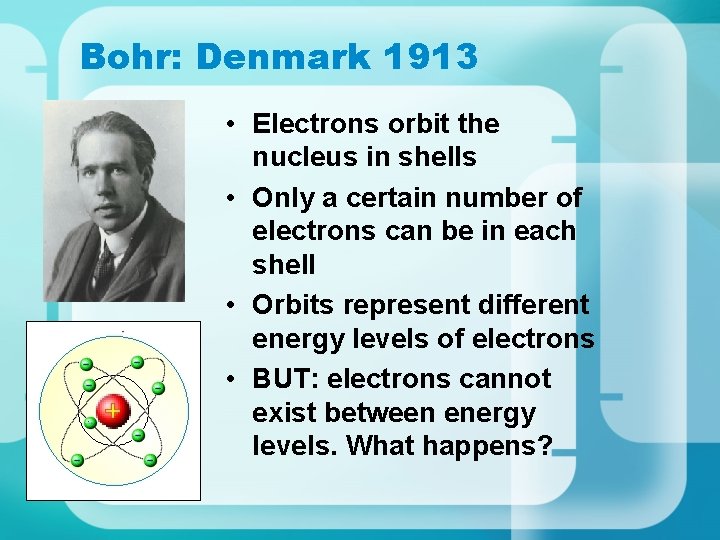
Bohr: Denmark 1913 • Electrons orbit the nucleus in shells • Only a certain number of electrons can be in each shell • Orbits represent different energy levels of electrons • BUT: electrons cannot exist between energy levels. What happens?

Light is a particle? • If light can have particle properties, and is a form of energy, maybe since electrons are particles with mass, maybe they can have energy properties • Since electrons cannot exist between energy levels, they only “jump” them, and get or give off energy. • We will get back to this in a few days!

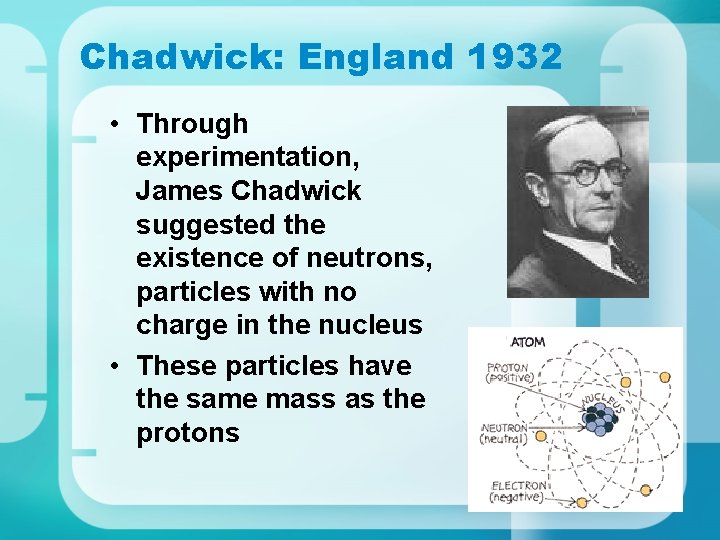
Chadwick: England 1932 • Through experimentation, James Chadwick suggested the existence of neutrons, particles with no charge in the nucleus • These particles have the same mass as the protons

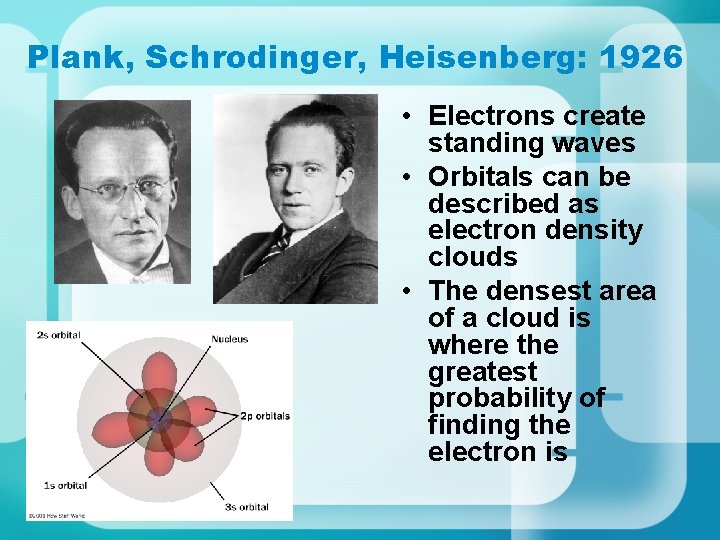
Plank, Schrodinger, Heisenberg: 1926 • Electrons create standing waves • Orbitals can be described as electron density clouds • The densest area of a cloud is where the greatest probability of finding the electron is

Table 1, p. 26

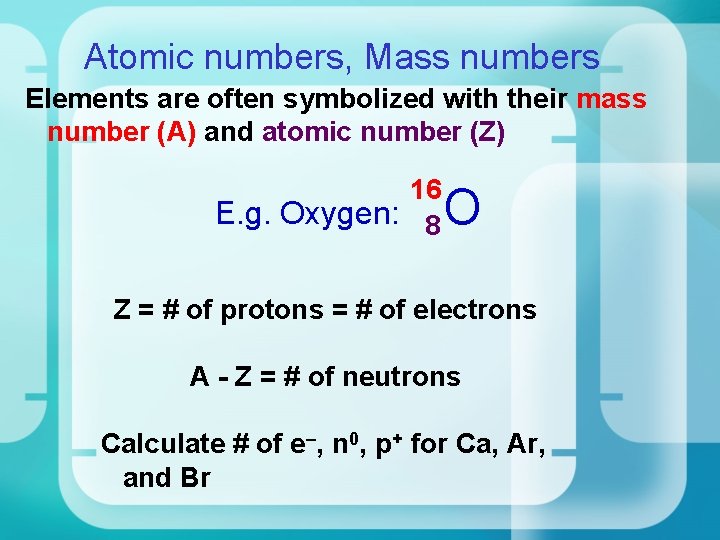
Atomic numbers, Mass numbers Elements are often symbolized with their mass number (A) and atomic number (Z) 16 E. g. Oxygen: 8 O Z = # of protons = # of electrons A - Z = # of neutrons Calculate # of e–, n 0, p+ for Ca, Ar, and Br

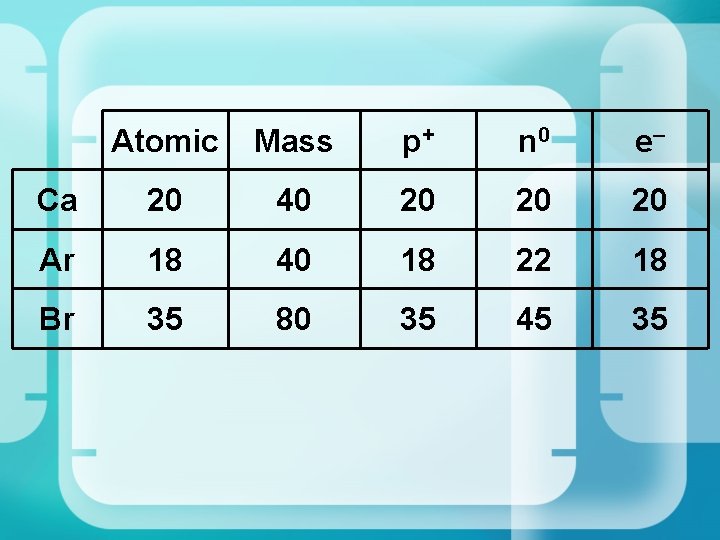
Atomic Mass p+ n 0 e– Ca 20 40 20 20 20 Ar 18 40 18 22 18 Br 35 80 35 45 35

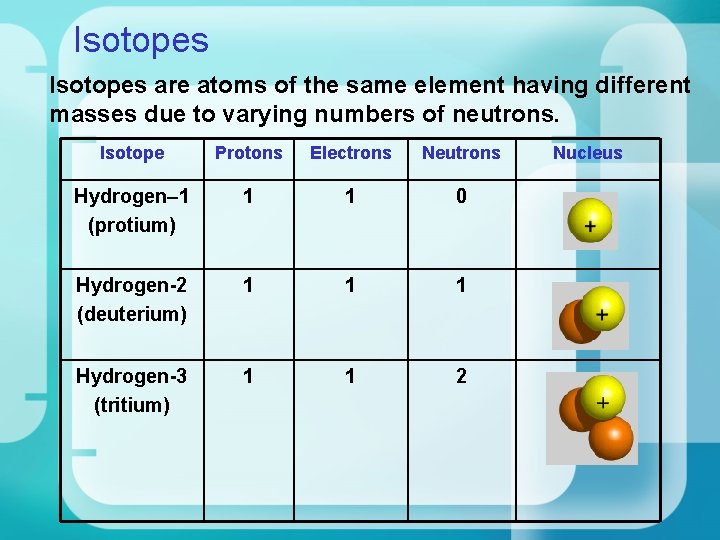
Isotopes are atoms of the same element having different masses due to varying numbers of neutrons. Isotope Protons Electrons Neutrons Hydrogen– 1 (protium) 1 1 0 Hydrogen-2 (deuterium) 1 1 1 Hydrogen-3 (tritium) 1 1 2 Nucleus

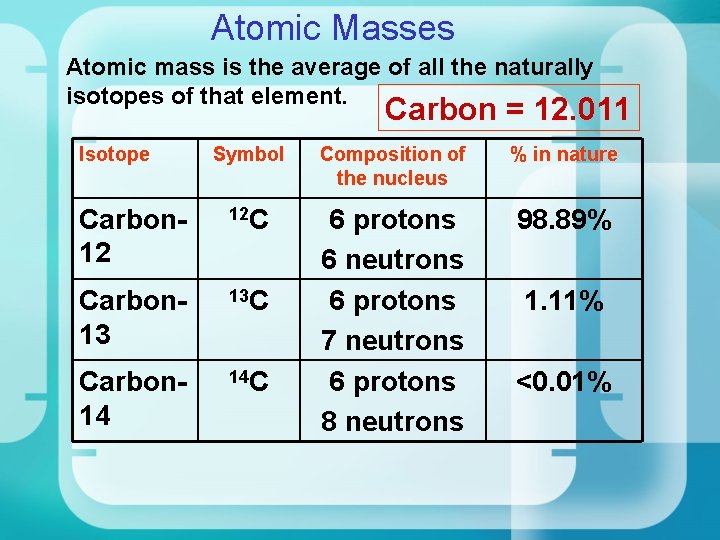
Atomic Masses Atomic mass is the average of all the naturally isotopes of that element. Carbon = 12. 011 Isotope Symbol Composition of the nucleus % in nature Carbon 12 12 C 98. 89% Carbon 13 13 C Carbon 14 14 C 6 protons 6 neutrons 6 protons 7 neutrons 6 protons 8 neutrons 1. 11% <0. 01%