Models of the Atom Greek Philosophers Greek philosophers














































- Slides: 78

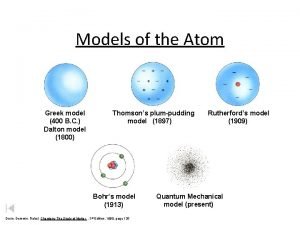
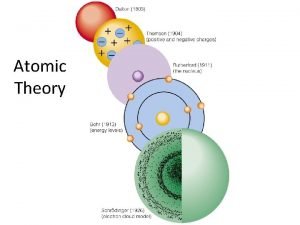
Models of the Atom

Greek Philosophers Greek philosophers were persons who thought about things, trying to understand the world and humankind’s place in it. The word, philosopher, comes from the Greek words, philos (to love) and sophia (wisdom). Philosophers are persons who try to become wise by careful thinking about things.

Greek Philosophers Greek philosophers thought and discussed ideas but did not do experiments.

Greek Philosophers Greek philosophers lived from 624 BC to 320 BC.

Is there a smallest piece of a substance? Greek philosophers debated whether a substance (like water) had a smallest piece or if you could keep dividing it up forever, finding no smallest piece of the substance.

Can you cut a substance in half indefinitely? Some Greek philosophers thought that you could keep cutting something in half, never coming to a smallest piece of the thing. Other Greek philosophers thought that if you kept cutting something in half, eventually you would come to the smallest piece of a thing that could not be cut in half.

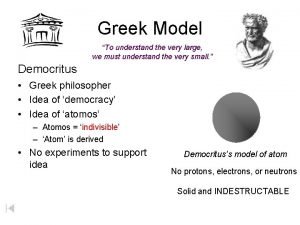
The Greek Philosopher, Democritus (460 BC) Democritus believed that if you cut a thing in half again and again, eventually you would find a small uncuttable particle of the thing. He called this thing the uncuttable (atomos in Greek) which is what the word, atom, means.

The Greek Philosopher, Democritus believed that every thing was made up of atoms and between the atoms was empty space. He thought atoms were very small which is why they can’t be seen. He argued that a beach viewed from a distance looks like one thing but close up it can be seen to be made up of grains of sand.

The Greek Philosopher, Democritus thought that all the properties of substances were due to the shapes and colours of the atoms that they were made of. He even believed that the soul was made up of soul atoms. Since he only believed in atoms (He was a materialist - only matter is real), Democritus was an atheist and did NOT believe in God. He also proposed the form of government called a Democracy.

The Greek Philosopher, Aristotle (384 BC) Aristotle, the teacher of Alexander the Great, taught that you could cut a thing in half forever, never coming to a smallest part. He believed that all substances were made up of four elements: earth, water, air and fire.

The Greek Philosopher, Aristotle argued against the idea of atoms and believed there was a god who was at the outside of the universe in a realm of perfection. He thought the earth was in a realm of imperfection in the centre of the universe.

A New Faith : The Way (Later Called Christianity) Jesus’ teachings were spread by His disciples after His death.

Christians Persecuted by Rome from 35 – 313 AD The early church was persecuted on and off from Jesus’ death to 313 AD.

Constantine Made Christianity Rome’s Favoured Religion Emperor Constantine with an army of 20, 000 fought an opponent, Maxentius’ army of 100, 000 at the battle of the Milvian bridge in 312 AD.

Constantine Made Christianity Rome’s Official Religion In a dream, Constantine (whose wife was a Christian) prayed for help and in his dream felt he would conquer if he put the first two letters of Christ’s Greek name (chi - X and Rho - P) on his soldier’s shields. In Christ’s name he would conquer! Constantine won the battle and made Christianity the favored religion in Rome by proclamation in 313 AD.

After Constantine, Rome Weakened As Rome lost influence, the church became stronger and preserved culture and learning. This time, the Middle Ages (400 – 1400 AD) is known as the Age of Faith.

Medieval Christians Studied Greek Philosophers The few Medieval Christians who were educated and who had access to Ancient manuscripts began reading Greek philosophers. They rejected Democritus’ atheism and so they also rejected his ideas of atoms. Since Aristotle believed in a god, Medieval Christians accepted Aristotle’s ideas.

Aristotle and Transmutation Aristotle taught that one substance could be changed into another by a process he called transmutation. During the Middle Ages, persons called alchemists believed in transmutation and tried to convert worthless substances (like lead) into valuable substances (like gold).

The Philosopher’s Stone Alchemists searched for the magical substance, the Philosopher’s stone, which was thought to be able to convert lead to gold and to give a person eternal life. Because alchemists used some occult practices (witchcraft) and thought that they could give themselves eternal life on their own, they were often suppressed by the Church.

Alchemists Develop New Equipment and Techniques Despite their fanciful and misguided ideas, Alchemists through experimentation developed many new techniques for purifying and separating mixtures. They also developed new equipment for experimenting like ovens, bellows, flasks, retorts, mortar and pestle, distilling equipment and glassblowing techniques.

Alchemists Discovered New Elements Alchemists also discovered some new elements like arsenic, antimony, bismuth and phosphorus.

Modern Atomic Theory : Robert Boyle (1627 -1691) wrote against Aristotle’s idea of four elements, writing that elements are “certain primitive and simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the ingredients of which all these perfectly mixt bodies are immediately compounded, and into which they are ultimately resolved. ”

Modern Atomic Theory : Robert Boyle (1627 -1691) Robert Boyle also appealed to all scientists to base their science on experiments only. Despite his forward, modern thinking, Boyle believed in Aristotle’s idea of transmutation.

Joseph Priestley (1733 -1804) • Joseph Priestley discovered the gas oxygen which he called dephlogisticated air. His experiments paved the way for Antoine Lavoisier to correctly explain what combustion (burning) is and its similarity to respiration (the combustion of food).

Henry Cavendish (1731 -1810) • Henry Cavendish discovered the gas hydrogen and noted that when hydrogen burns in air it produces water. Thus Cavendish showed that water was a compound not an element like Aristotle taught.

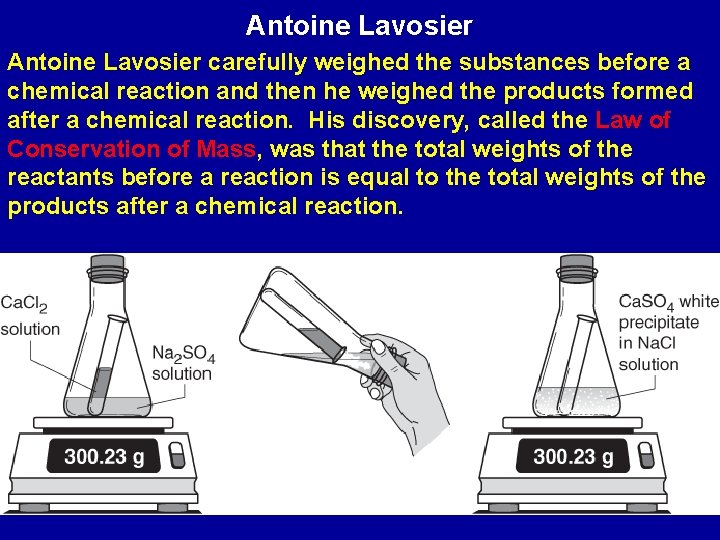
Antoine Lavoisier : Brilliant Scientist (1743 -1794) Antoine Lavoisier discovered many laws of chemistry and helped develop the metric system (SI – System Internationale)

Antoine Lavosier repeated Priestley’s experiments, naming the newly found gas, oxygen. He correctly explained that combustion (burning) involves the combination or addition of oxygen to a burning substance rather than the loss of phlogiston, an earlier incorrect theory. Oxygen is added to a substance that burns.

Combustion is Always a Combination with Oxygen 1. 2. 3. Wood + oxygen carbon dioxide (gas) + water + ash Food + oxygen carbon dioxide + water Gasoline + oxygen carbon dioxide + water

Antoine Lavosier carefully weighed the substances before a chemical reaction and then he weighed the products formed after a chemical reaction. His discovery, called the Law of Conservation of Mass, was that the total weights of the reactants before a reaction is equal to the total weights of the products after a chemical reaction.

Burning Wood : The Law of Conservation of Mass • If a person weighs just the wood before it burns and the ash after it burns, it seems like something has been lost. • But if all reactants and all products are weighed, then the total weight before equals the total weight after.

Antoine Lavosier believed in freedom, equality and brotherhood which were the ideals of the French Revolution. However since he came from a rich family, the Revolutionary government did not trust him and unfortunately had him beheaded.

John Dalton : “Father” of the Modern Atomic Theory John Dalton, a Quaker school teacher, started to develop his ideas about atoms in 1802 but it was not until 1827 that he published a full account of his atomic theory.

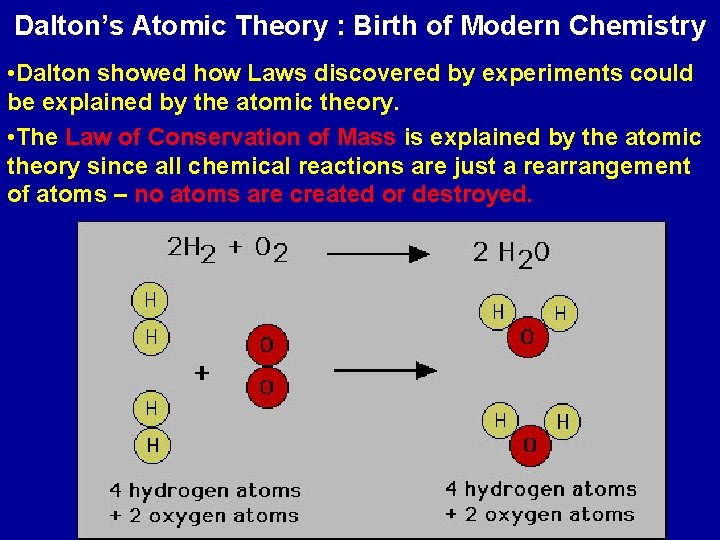
Dalton’s Atomic Theory : Birth of Modern Chemistry • Most of the ideas of Dalton’s atomic theory were not new. Greeks like Democritus and most scientists after Robert Boyle philosophically (in thought) agreed on the main points about atoms.

Dalton’s Atomic Theory : Birth of Modern Chemistry • Dalton showed how Laws discovered by experiments could be explained by the atomic theory. • The Law of Conservation of Mass is explained by the atomic theory since all chemical reactions are just a rearrangement of atoms – no atoms are created or destroyed.

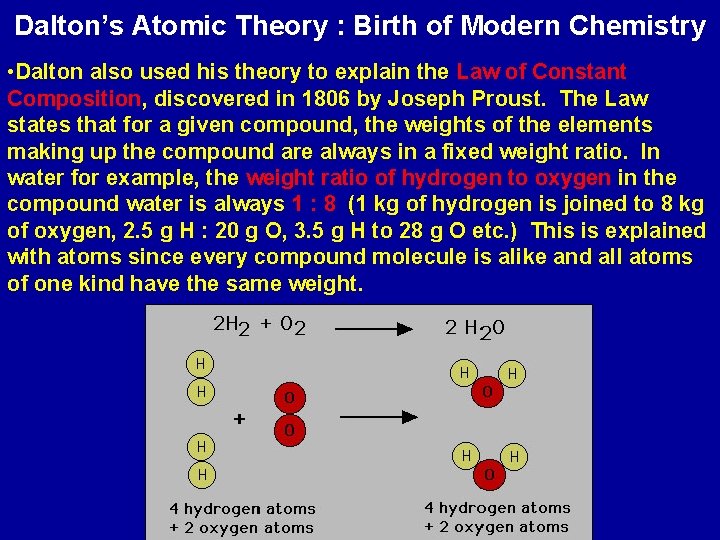
Dalton’s Atomic Theory : Birth of Modern Chemistry • Dalton also used his theory to explain the Law of Constant Composition, discovered in 1806 by Joseph Proust. The Law states that for a given compound, the weights of the elements making up the compound are always in a fixed weight ratio. In water for example, the weight ratio of hydrogen to oxygen in the compound water is always 1 : 8 (1 kg of hydrogen is joined to 8 kg of oxygen, 2. 5 g H : 20 g O, 3. 5 g H to 28 g O etc. ) This is explained with atoms since every compound molecule is alike and all atoms of one kind have the same weight.

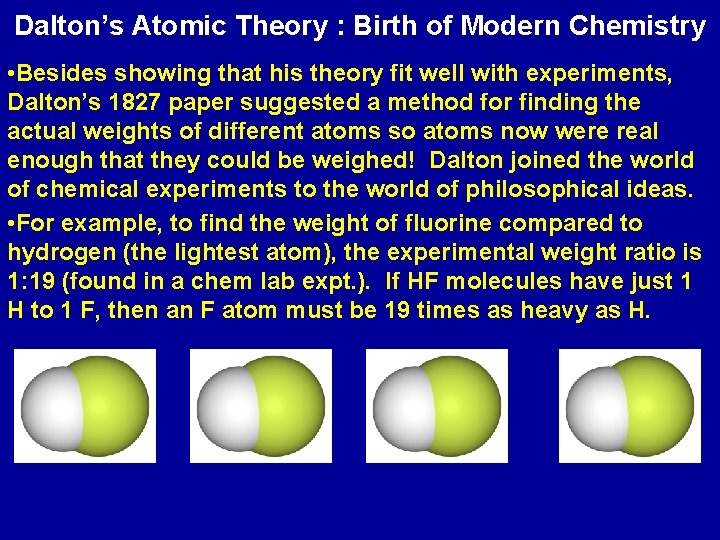
Dalton’s Atomic Theory : Birth of Modern Chemistry • Besides showing that his theory fit well with experiments, Dalton’s 1827 paper suggested a method for finding the actual weights of different atoms so atoms now were real enough that they could be weighed! Dalton joined the world of chemical experiments to the world of philosophical ideas. • For example, to find the weight of fluorine compared to hydrogen (the lightest atom), the experimental weight ratio is 1: 19 (found in a chem lab expt. ). If HF molecules have just 1 H to 1 F, then an F atom must be 19 times as heavy as H.

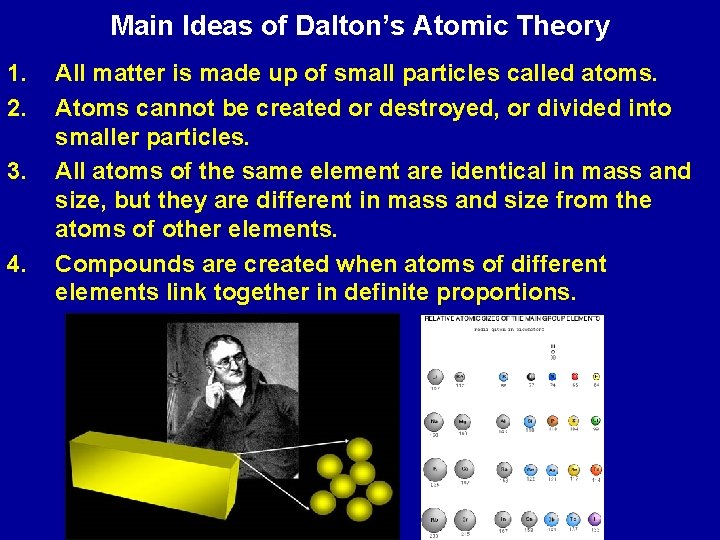
Main Ideas of Dalton’s Atomic Theory 1. 2. 3. 4. All matter is made up of small particles called atoms. Atoms cannot be created or destroyed, or divided into smaller particles. All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements. Compounds are created when atoms of different elements link together in definite proportions.

Atoms are real – not just ideas (They have unique weights that can be experimentally determined)! Thanks, J. D.

John Dalton’s Model of the Atom (1827) • Dalton like the Greeks thought that atoms were hard indivisible, uncuttable particles, solid like billiard balls.

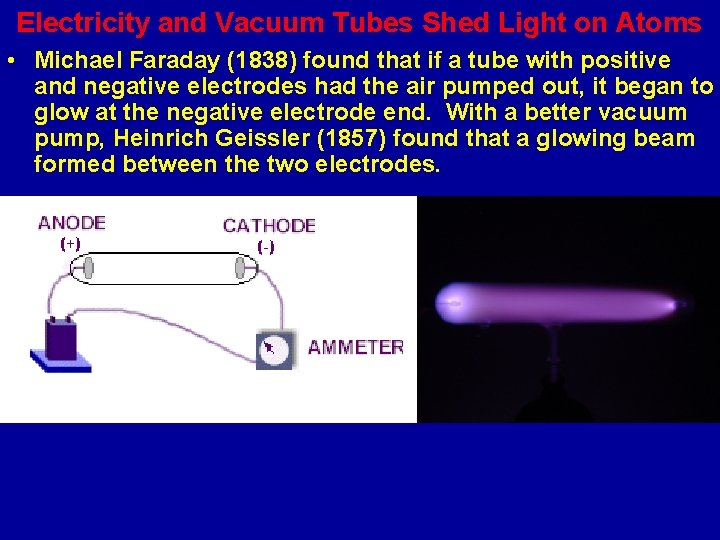
Electricity and Vacuum Tubes Shed Light on Atoms • Michael Faraday (1838) found that if a tube with positive and negative electrodes had the air pumped out, it began to glow at the negative electrode end. With a better vacuum pump, Heinrich Geissler (1857) found that a glowing beam formed between the two electrodes.

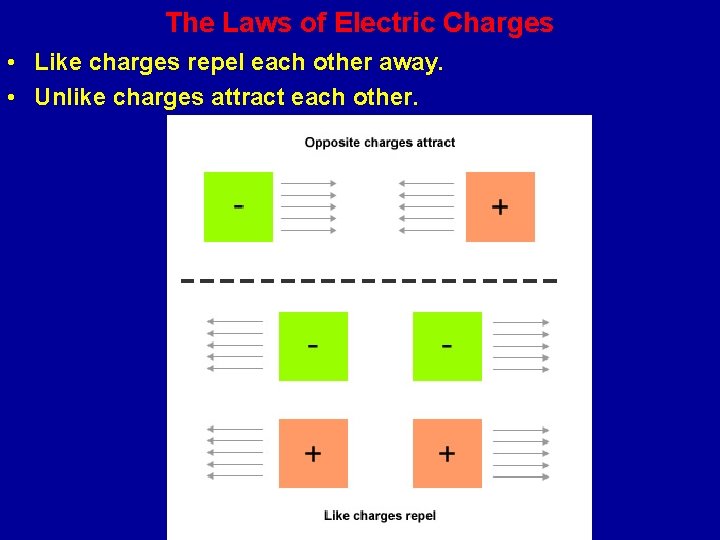
The Laws of Electric Charges • Like charges repel each other away. • Unlike charges attract each other.

Where is the glowing beam coming from? • Experiments by William Crookes (1870 s) and J. J. Thompson (1897) showed that the glowing beam was coming from the negative electrode (cathode) and that the glowing beam was negatively charged. The glowing beam was called cathode rays because it came from the cathode.

The Cathode Rays Are Negative Particles • Since the cathode rays could rotate a paddlewheel inside a cathode ray tube, experimenters thought they must be made up of negative particles which came to be called electrons (from the Greek word for amber, elektron).

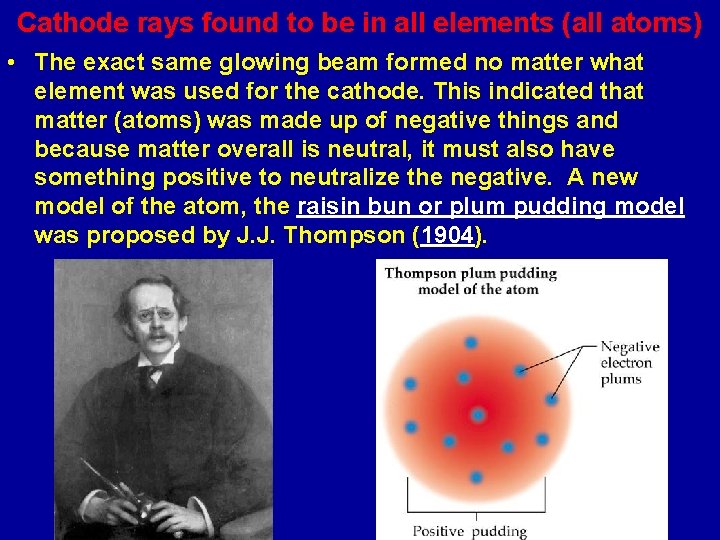
Cathode rays found to be in all elements (all atoms) • The exact same glowing beam formed no matter what element was used for the cathode. This indicated that matter (atoms) was made up of negative things and because matter overall is neutral, it must also have something positive to neutralize the negative. A new model of the atom, the raisin bun or plum pudding model was proposed by J. J. Thompson (1904).

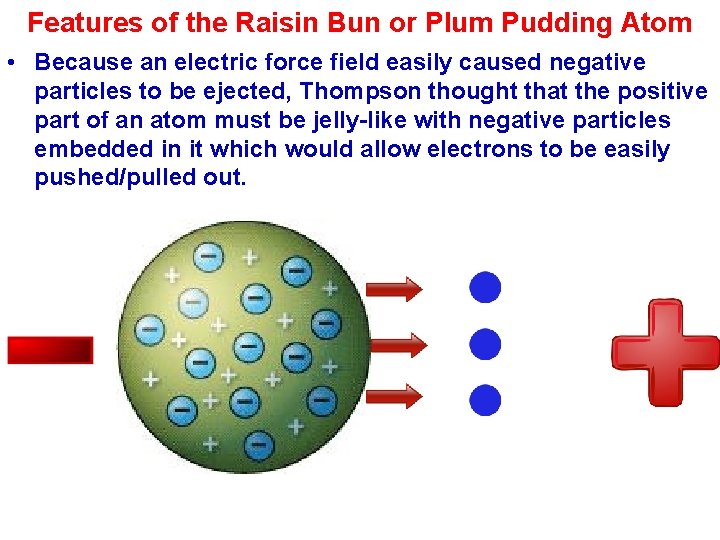
Features of the Raisin Bun or Plum Pudding Atom • Because an electric force field easily caused negative particles to be ejected, Thompson thought that the positive part of an atom must be jelly-like with negative particles embedded in it which would allow electrons to be easily pushed/pulled out.

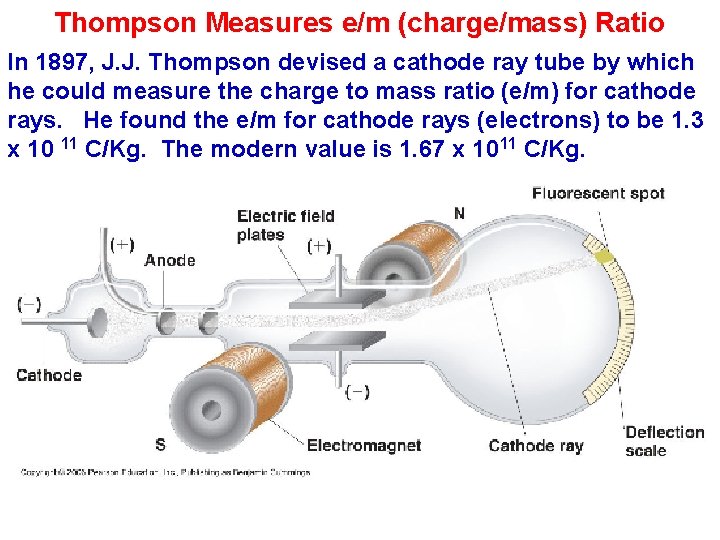
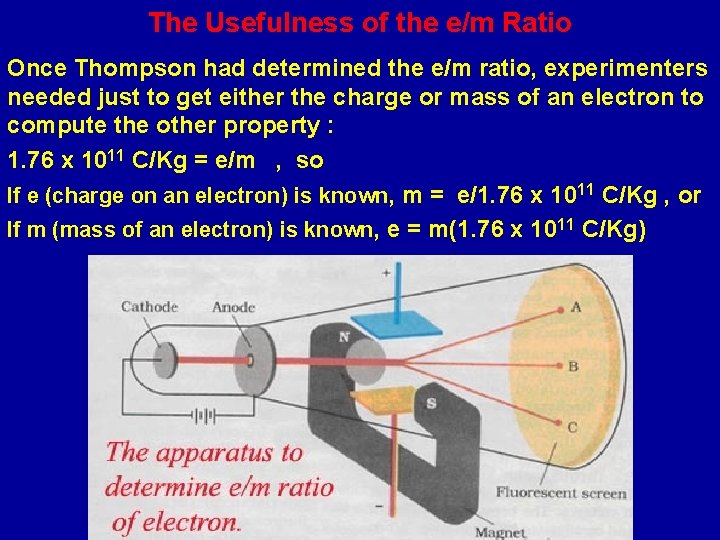
Thompson Measures e/m (charge/mass) Ratio In 1897, J. J. Thompson devised a cathode ray tube by which he could measure the charge to mass ratio (e/m) for cathode rays. He found the e/m for cathode rays (electrons) to be 1. 3 x 10 11 C/Kg. The modern value is 1. 67 x 1011 C/Kg.

The Usefulness of the e/m Ratio Once Thompson had determined the e/m ratio, experimenters needed just to get either the charge or mass of an electron to compute the other property : 1. 76 x 1011 C/Kg = e/m , so If e (charge on an electron) is known, m = e/1. 76 x 1011 C/Kg , or If m (mass of an electron) is known, e = m(1. 76 x 1011 C/Kg)

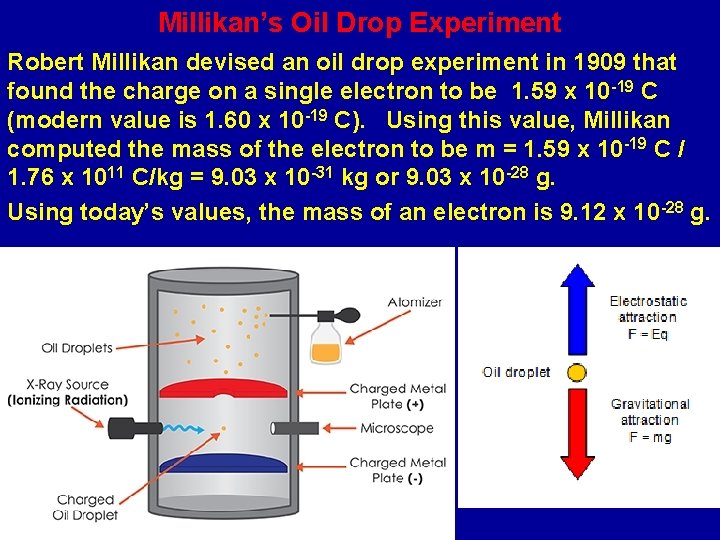
Millikan’s Oil Drop Experiment Robert Millikan devised an oil drop experiment in 1909 that found the charge on a single electron to be 1. 59 x 10 -19 C (modern value is 1. 60 x 10 -19 C). Using this value, Millikan computed the mass of the electron to be m = 1. 59 x 10 -19 C / 1. 76 x 1011 C/kg = 9. 03 x 10 -31 kg or 9. 03 x 10 -28 g. Using today’s values, the mass of an electron is 9. 12 x 10 -28 g.

How Millikan Found the Charge on an Electron Robert Millikan radiated air (knocking out electrons) that had small oil droplets that picked up some of these electrons. A hole in an upper plate allowed some oil droplets to fall through. Charges on the upper and lower plates could be adjusted to suspend the oil droplets. There was a minimum charge difference to suspend the droplets and this was the charge on an electron.

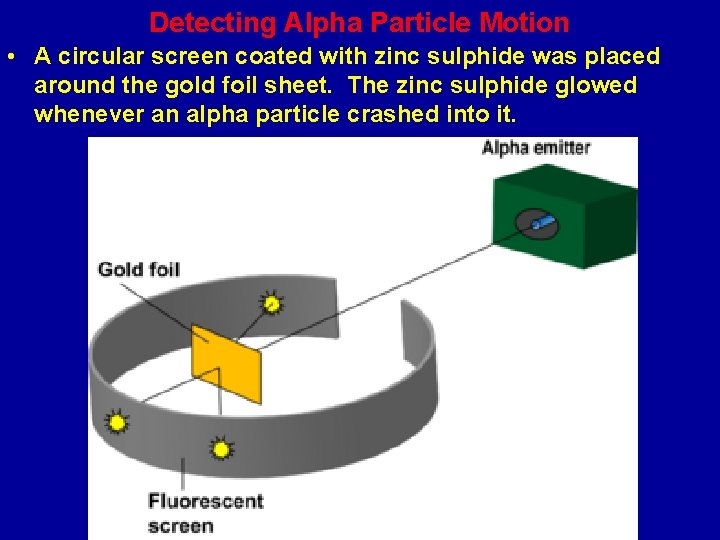
The Gold Foil Experiment and The Nuclear Atom • Ernest Rutherford designed an experiment in which a beam of heavy positive alpha particles was shot at a very thin gold foil sheet that was only a few atoms thick. Rutherford expected that the alpha particles would plow directly through the gold atoms (supposedly soft plum pudding atoms) like an artillery shell would plow through a piece of tissue paper.

Detecting Alpha Particle Motion • A circular screen coated with zinc sulphide was placed around the gold foil sheet. The zinc sulphide glowed whenever an alpha particle crashed into it.

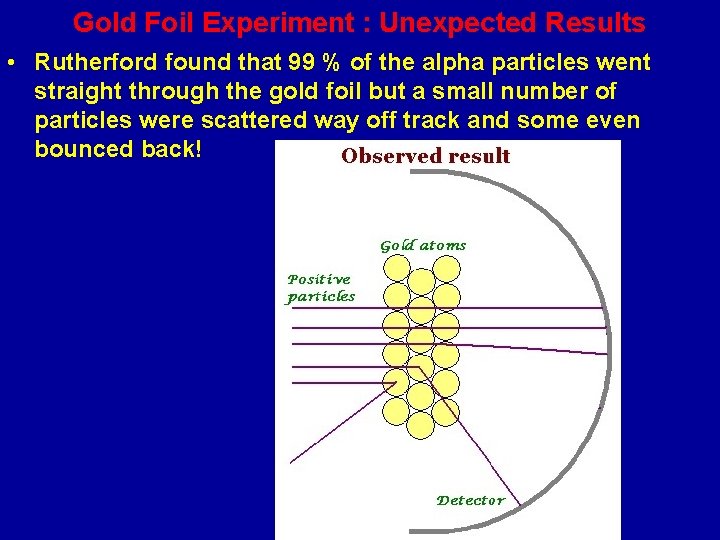
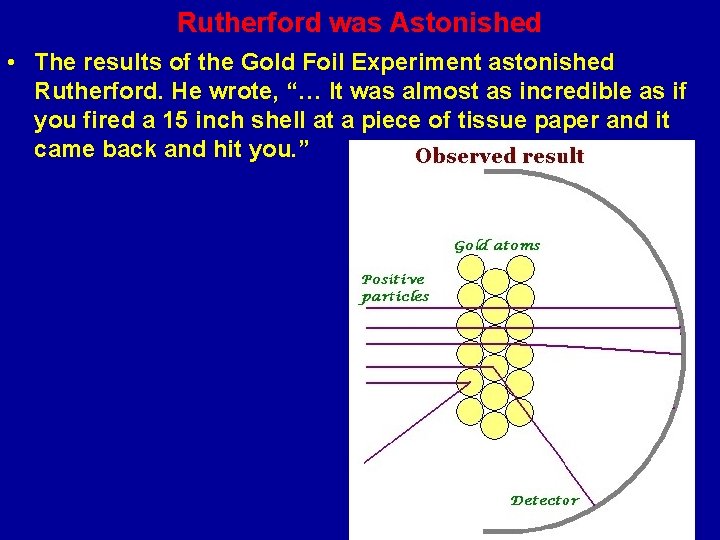
Gold Foil Experiment : Unexpected Results • Rutherford found that 99 % of the alpha particles went straight through the gold foil but a small number of particles were scattered way off track and some even bounced back!

Rutherford was Astonished • The results of the Gold Foil Experiment astonished Rutherford. He wrote, “… It was almost as incredible as if you fired a 15 inch shell at a piece of tissue paper and it came back and hit you. ”

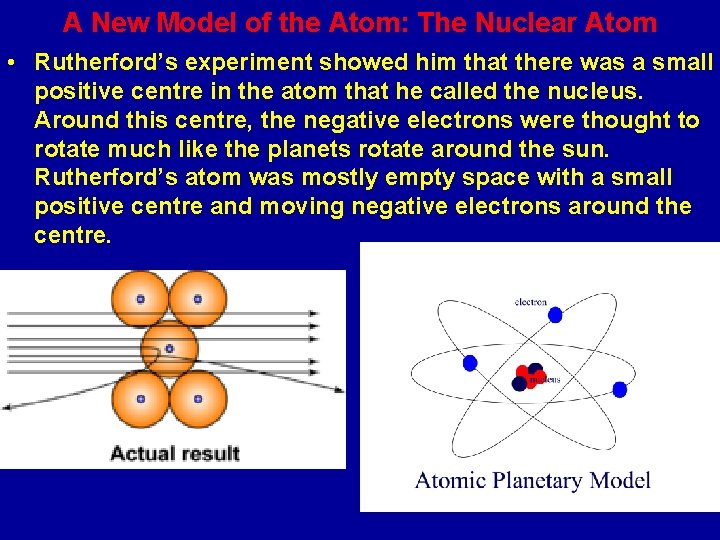
A New Model of the Atom: The Nuclear Atom • Rutherford’s experiment showed him that there was a small positive centre in the atom that he called the nucleus. Around this centre, the negative electrons were thought to rotate much like the planets rotate around the sun. Rutherford’s atom was mostly empty space with a small positive centre and moving negative electrons around the centre.

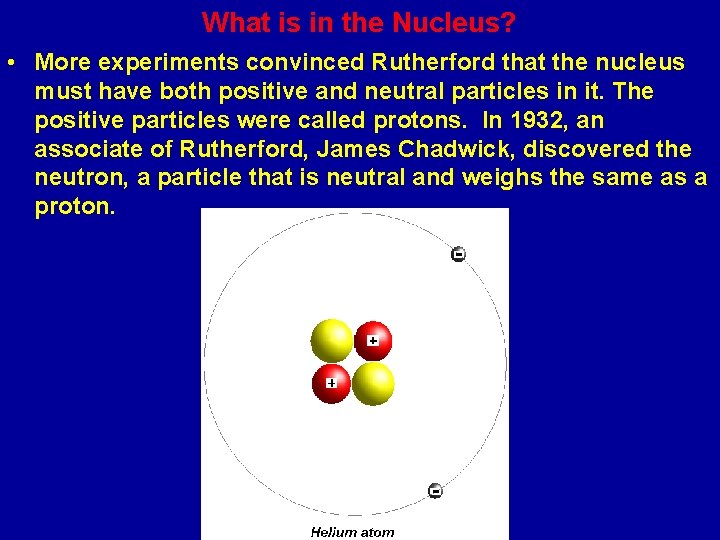
What is in the Nucleus? • More experiments convinced Rutherford that the nucleus must have both positive and neutral particles in it. The positive particles were called protons. In 1932, an associate of Rutherford, James Chadwick, discovered the neutron, a particle that is neutral and weighs the same as a proton.

Atom Composition: Three Particles 1. 2. 3. 4. 5. 6. Atoms are composed of protons, neutrons and electrons. The nucleus contains protons (+) and neutrons (0). Electrons (-) are moving rapidly around the nucleus. The number of electrons equals the number of protons in a neutral atom. The charge on one electron exactly balances the charge on one proton. 1+ added to 1 gives a charge of 0. A proton and neutron weigh 1832 X as much as an electron.

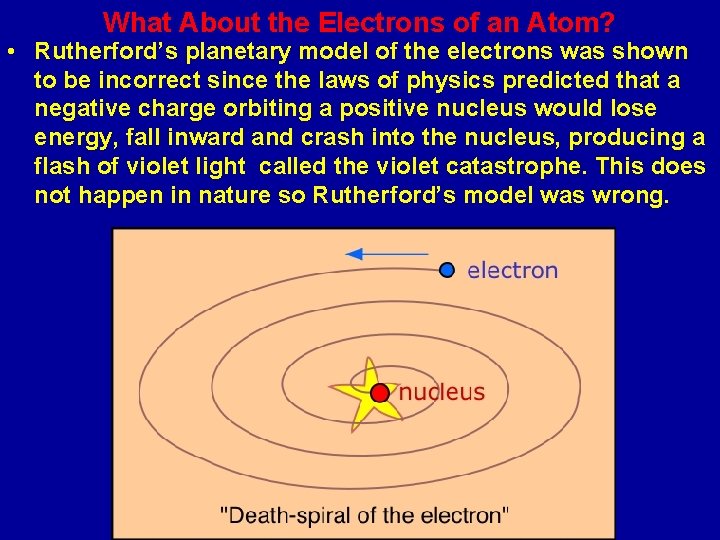
What About the Electrons of an Atom? • Rutherford’s planetary model of the electrons was shown to be incorrect since the laws of physics predicted that a negative charge orbiting a positive nucleus would lose energy, fall inward and crash into the nucleus, producing a flash of violet light called the violet catastrophe. This does not happen in nature so Rutherford’s model was wrong.

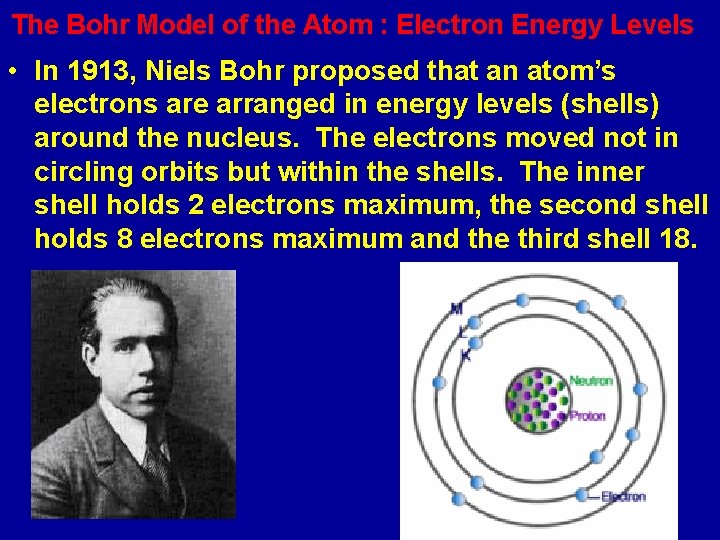
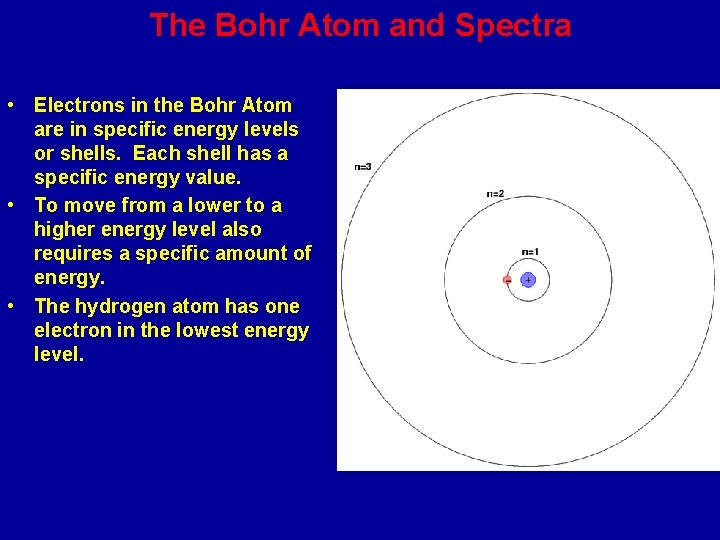
The Bohr Model of the Atom : Electron Energy Levels • In 1913, Niels Bohr proposed that an atom’s electrons are arranged in energy levels (shells) around the nucleus. The electrons moved not in circling orbits but within the shells. The inner shell holds 2 electrons maximum, the second shell holds 8 electrons maximum and the third shell 18.

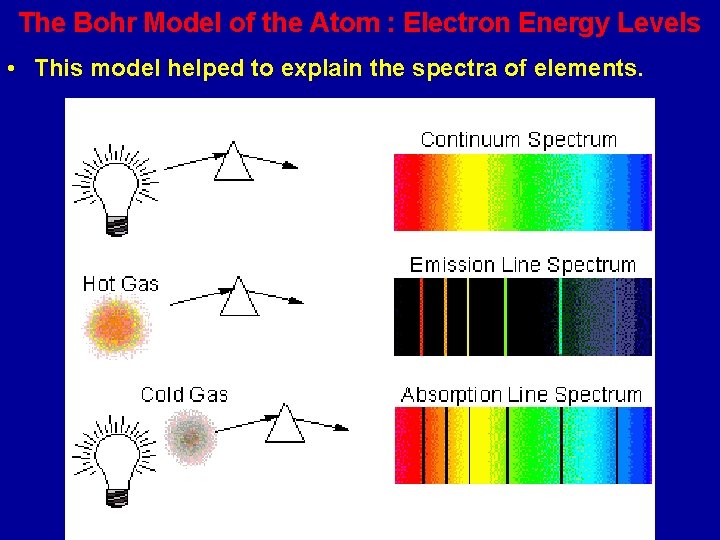
The Bohr Model of the Atom : Electron Energy Levels • This model helped to explain the spectra of elements.

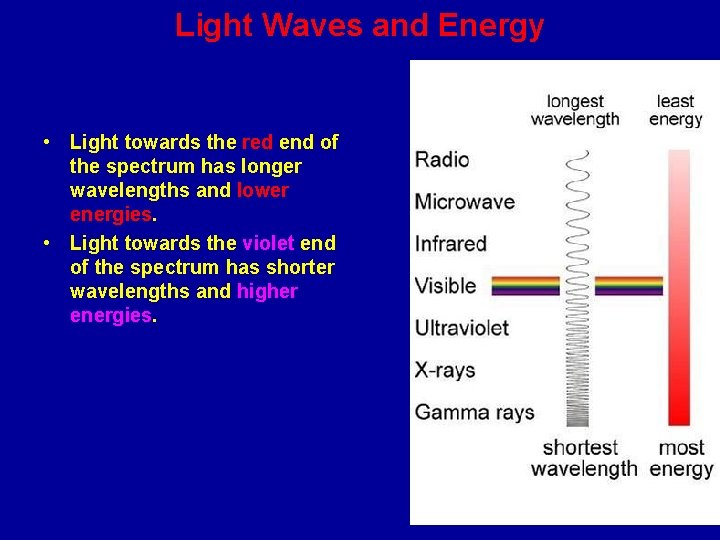
Light Waves and Wavelength • White light is a mixture of many colours of light : ROYGBIV (red, orange, yellow, green, blue, indigo, violet). • The different colours of light have different wavelengths. • Violet light has shorter wavelength while red light has longer wavelength.

Light Waves and Energy • Light towards the red end of the spectrum has longer wavelengths and lower energies. • Light towards the violet end of the spectrum has shorter wavelengths and higher energies.

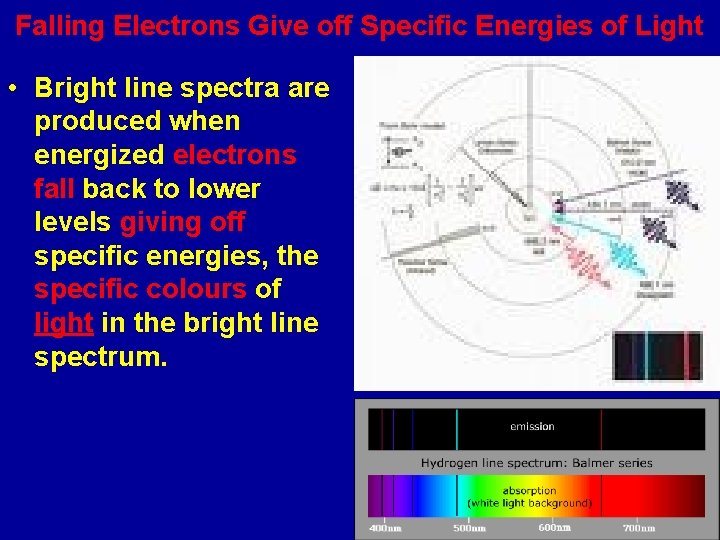
Bright and Dark Line Spectra • When an element is energized (by heating it or passing electricity through it), it gives off a spectrum which is made up of just specific energies of light. • When white light (made up of all light energies) is passed through a cool gas of an element, a spectrum with dark lines is produced.

Element Spectra : Unique Like Fingerprints • Each element has its own unique bright line and absorption spectrum which identify it like different fingerprints identify different people.

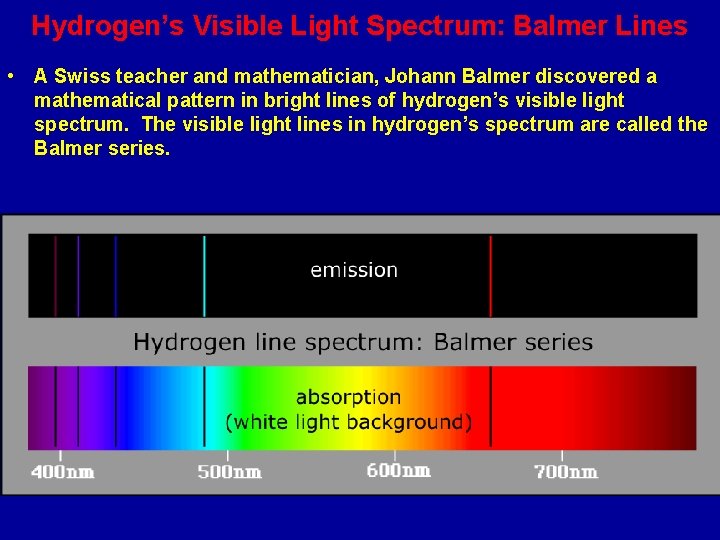
Hydrogen’s Visible Light Spectrum: Balmer Lines • A Swiss teacher and mathematician, Johann Balmer discovered a mathematical pattern in bright lines of hydrogen’s visible light spectrum. The visible light lines in hydrogen’s spectrum are called the Balmer series.

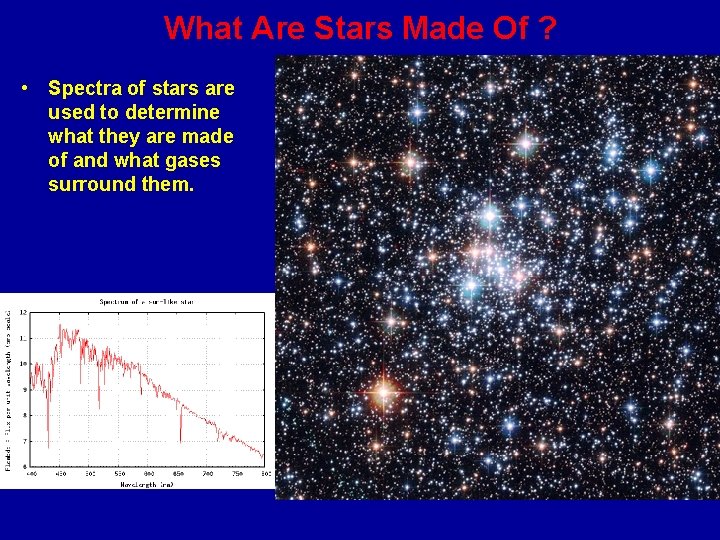
What Are Stars Made Of ? • Spectra of stars are used to determine what they are made of and what gases surround them.

The Bohr Atom and Spectra • Electrons in the Bohr Atom are in specific energy levels or shells. Each shell has a specific energy value. • To move from a lower to a higher energy level also requires a specific amount of energy. • The hydrogen atom has one electron in the lowest energy level.

The Bohr Atom and Spectra • Electrons can move up to higher energy levels if energy is added to them. These electrons are said to be excited. • When electrons drop to lower energy levels (due to their attraction to the nucleus {- and + charges}), they release or give off energy.

Falling Electrons Give off Specific Energies of Light • Bright line spectra are produced when energized electrons fall back to lower levels giving off specific energies, the specific colours of light in the bright line spectrum.

The Bohr Model of the Atom : Main Ideas 1. 2. 3. 4. 5. The nucleus is just 1/10, 000 of the atom’s volume. Most of the atom is empty space. The nucleus contains 99. 9% of an atom’s mass/weight. The nucleus contains positive protons and neutral neutrons which are 1832 times heavier than electrons. The negative electrons are found in energy shells around the nucleus. The number of electrons is always equal to the number of protons

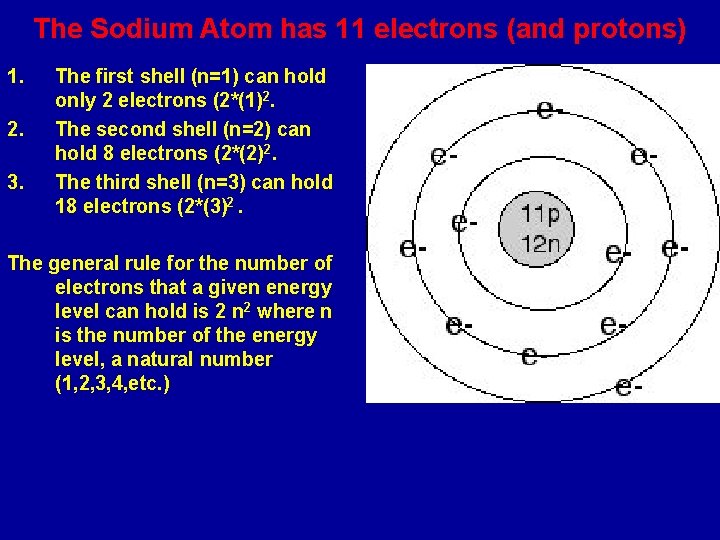
The Sodium Atom has 11 electrons (and protons) 1. 2. 3. The first shell (n=1) can hold only 2 electrons (2*(1)2. The second shell (n=2) can hold 8 electrons (2*(2)2. The third shell (n=3) can hold 18 electrons (2*(3)2. The general rule for the number of electrons that a given energy level can hold is 2 n 2 where n is the number of the energy level, a natural number (1, 2, 3, 4, etc. )

Visualizing an Atom If an atom were blown up in size to the size of a football field, the nucleus (protons and neutrons) would be the size of a single pea on the 50 m (yd) line and the electrons would be like salt grains buzzing around the field in their shells.

How Small is an Atom? • In an “average” grain of salt from a salt shaker, there are 1. 2 X 10 18 atoms or 1, 200, 000, 000 atoms.

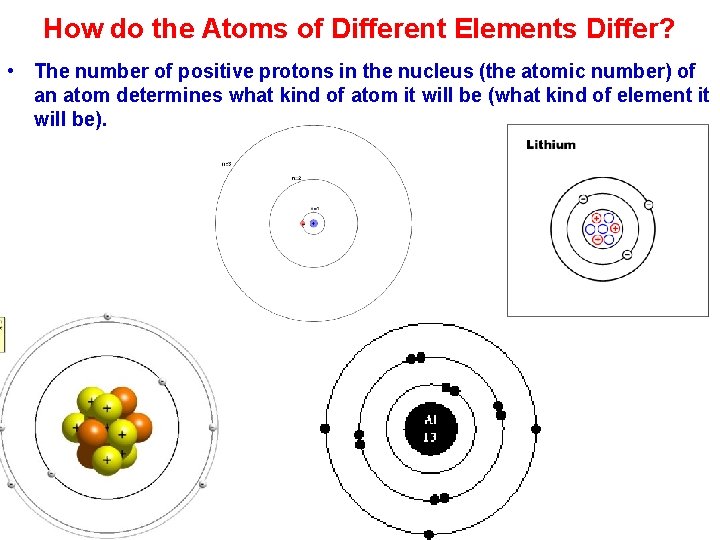
How do the Atoms of Different Elements Differ? • The number of positive protons in the nucleus (the atomic number) of an atom determines what kind of atom it will be (what kind of element it will be).

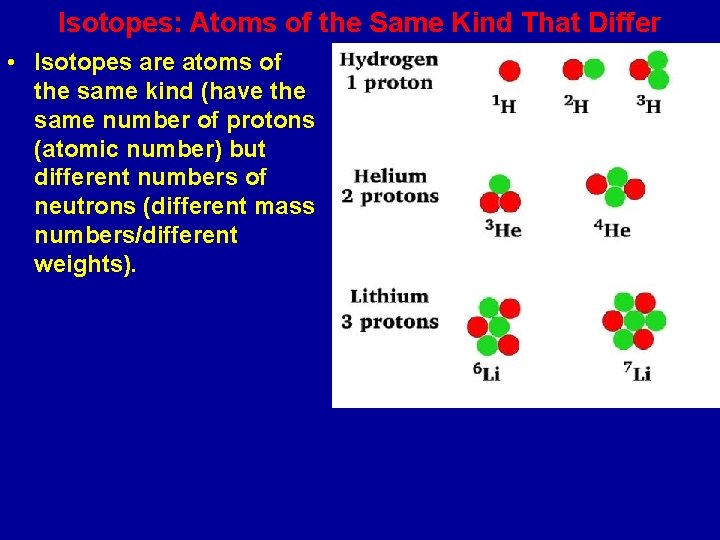
Isotopes: Atoms of the Same Kind That Differ • Isotopes are atoms of the same kind (have the same number of protons (atomic number) but different numbers of neutrons (different mass numbers/different weights).

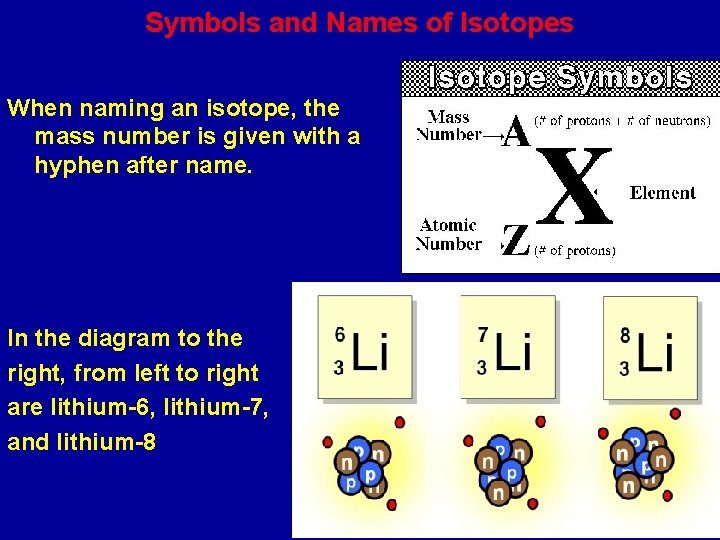
Symbols and Names of Isotopes When naming an isotope, the mass number is given with a hyphen after name. In the diagram to the right, from left to right are lithium-6, lithium-7, and lithium-8

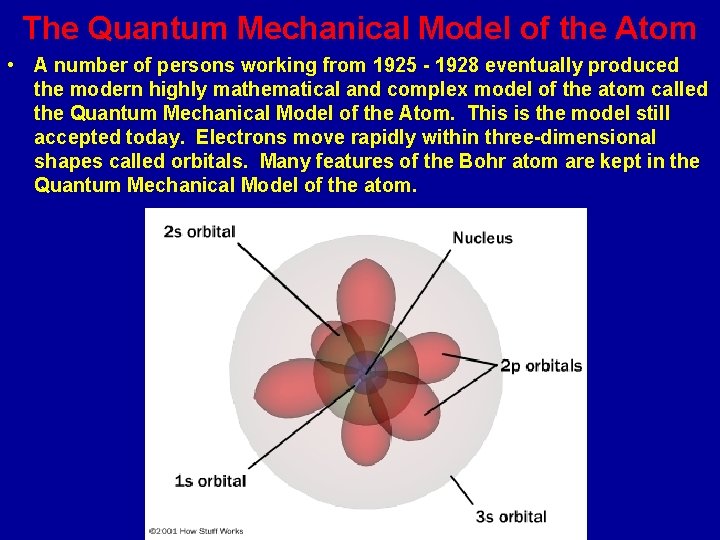
The Quantum Mechanical Model of the Atom • A number of persons working from 1925 - 1928 eventually produced the modern highly mathematical and complex model of the atom called the Quantum Mechanical Model of the Atom. This is the model still accepted today. Electrons move rapidly within three-dimensional shapes called orbitals. Many features of the Bohr atom are kept in the Quantum Mechanical Model of the atom.

Review of the Models of the Atom from 1800 - 1940

 Objective of physical education in athens greece
Objective of physical education in athens greece Ancient greek philosophers final jeopardy
Ancient greek philosophers final jeopardy 3 greek philosophers
3 greek philosophers Early greek philosophers in psychology
Early greek philosophers in psychology Greek philosophers ethics
Greek philosophers ethics Big 3 greek philosophers
Big 3 greek philosophers Greek philosophers worksheet
Greek philosophers worksheet Kelemahan teori dalton
Kelemahan teori dalton The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom John dalton billiard ball model
John dalton billiard ball model Lesson 11 atomic pudding models of the atom
Lesson 11 atomic pudding models of the atom History of the atom
History of the atom History of atomic models timeline
History of atomic models timeline Atomic alchemy
Atomic alchemy What are modals and semi modals
What are modals and semi modals Affility
Affility Greek model of atom
Greek model of atom Ancient greek model of atom
Ancient greek model of atom Atoms in greek
Atoms in greek Sino si baron de montesquieu
Sino si baron de montesquieu Light is
Light is Realism philosophy in education
Realism philosophy in education Event philosophy
Event philosophy Monitor solution to dining philosophers
Monitor solution to dining philosophers Philosophers of industrialization
Philosophers of industrialization Kierkegaard antihegelismo
Kierkegaard antihegelismo Dining philosophers problem using monitors
Dining philosophers problem using monitors Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lời thề hippocrates
Lời thề hippocrates Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu điện thế nghỉ
điện thế nghỉ Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Gấu đi như thế nào
Gấu đi như thế nào Lp html
Lp html Thế nào là số nguyên tố
Thế nào là số nguyên tố Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Một số thể thơ truyền thống
Một số thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Tư thế worm breton
Tư thế worm breton Bổ thể
Bổ thể ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng The greek miracle
The greek miracle Jari jari atom
Jari jari atom Atom nedr
Atom nedr Atom jj thomson
Atom jj thomson Atom elementleri ve sembolleri
Atom elementleri ve sembolleri Atom central
Atom central Io2f lewis structure
Io2f lewis structure Which scientist discovered neutron
Which scientist discovered neutron Periodic trends definition
Periodic trends definition Nomor atom mo
Nomor atom mo