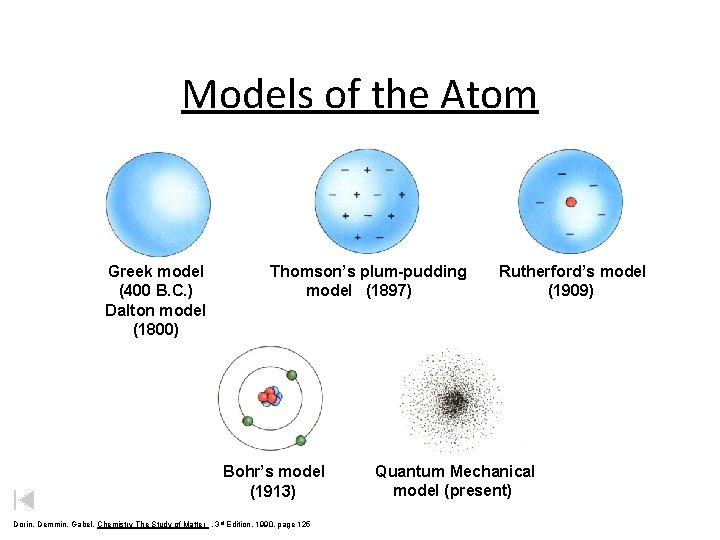
Models of the Atom Daltons Greek model 400
![Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h/013696a914218dcf3e98e6017ba23ad0/image-21.jpg)
![Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h/013696a914218dcf3e98e6017ba23ad0/image-22.jpg)


- Slides: 39

Models of the Atom Dalton’s Greek model (400 (1803) B. C. ) Dalton model (1800) Thomson’s plum-pudding model (1897) Bohr’s model (1913) Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 125 Rutherford’s model (1909) Quantum Mechanical model (present)

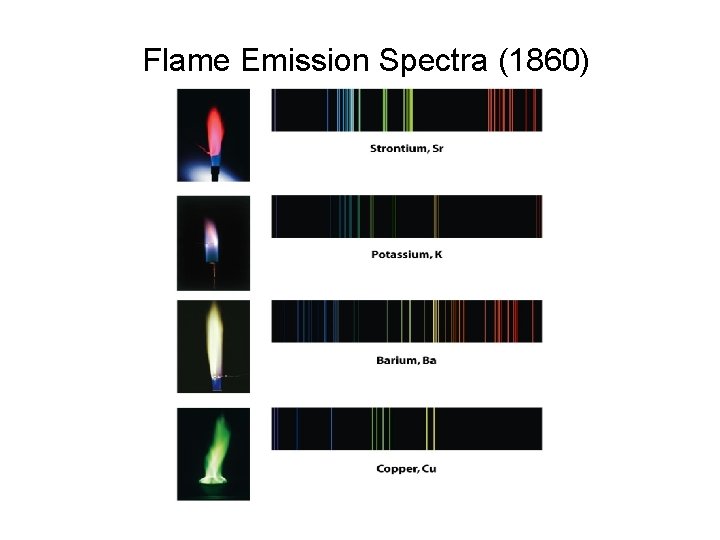
Flame Emission Spectra (1860)

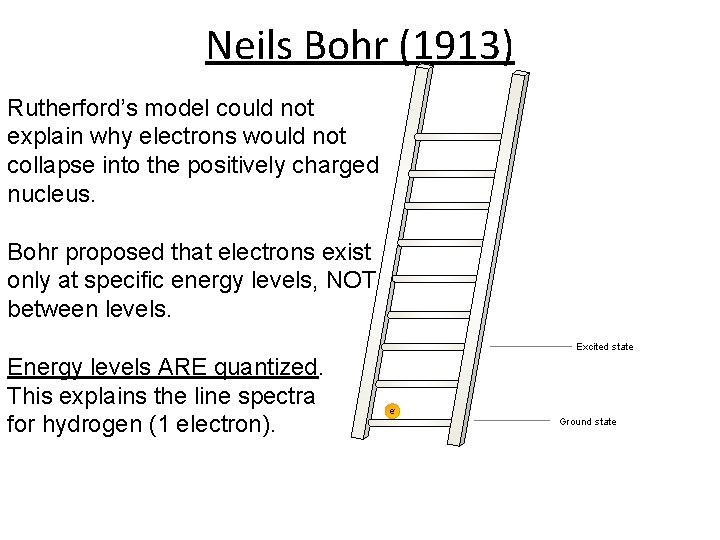
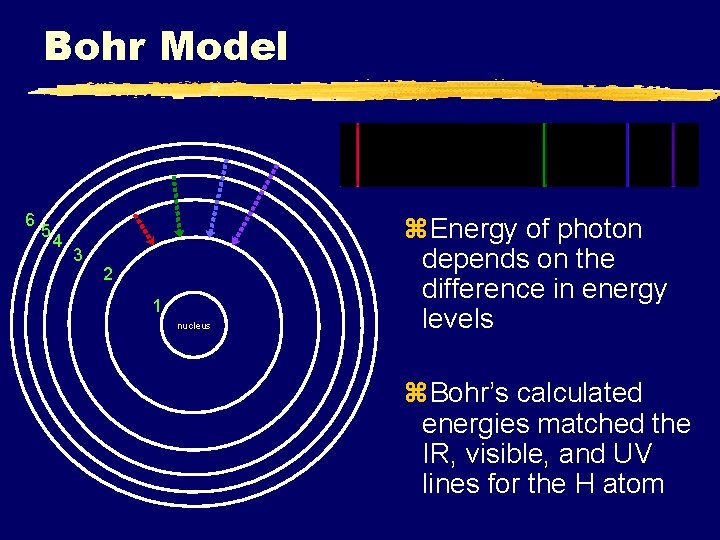
Neils Bohr (1913) Rutherford’s model could not explain why electrons would not collapse into the positively charged nucleus. Bohr proposed that electrons exist only at specific energy levels, NOT between levels. Excited state Energy levels ARE quantized. This explains the line spectra for hydrogen (1 electron). e- Ground state

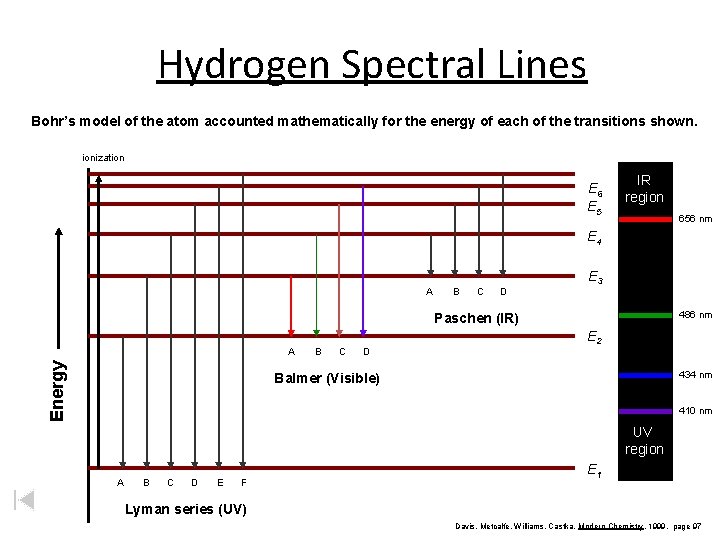
Hydrogen Spectral Lines Bohr’s model of the atom accounted mathematically for the energy of each of the transitions shown. ionization E 6 E 5 IR region 656 nm E 4 A B C D E 3 486 nm Paschen (IR) Energy A B C D E 2 434 nm Balmer (Visible) 410 nm UV region A B C D E F E 1 Lyman series (UV) Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 97

Bohr Model 6 5 4 3 2 1 nucleus z. Energy of photon depends on the difference in energy levels z. Bohr’s calculated energies matched the IR, visible, and UV lines for the H atom

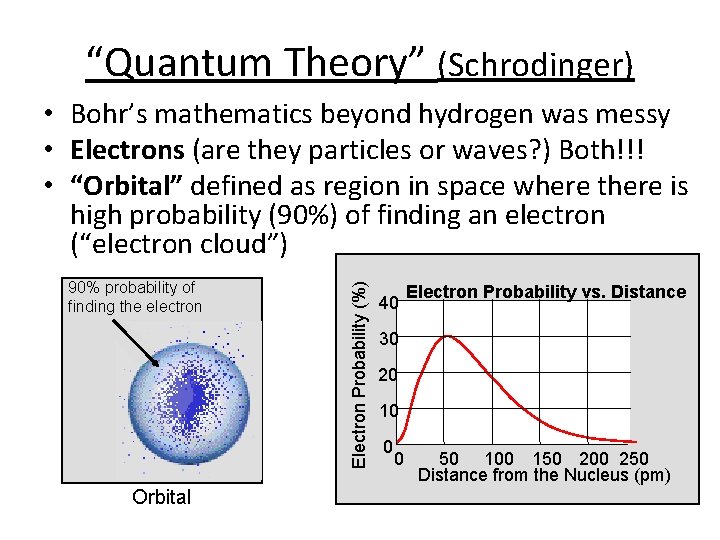
“Quantum Theory” (Schrodinger) 90% probability of finding the electron Orbital Electron Probability (%) • Bohr’s mathematics beyond hydrogen was messy • Electrons (are they particles or waves? ) Both!!! • “Orbital” defined as region in space where there is high probability (90%) of finding an electron (“electron cloud”) 40 Electron Probability vs. Distance 30 20 10 0 0 50 100 150 200 250 Distance from the Nucleus (pm)

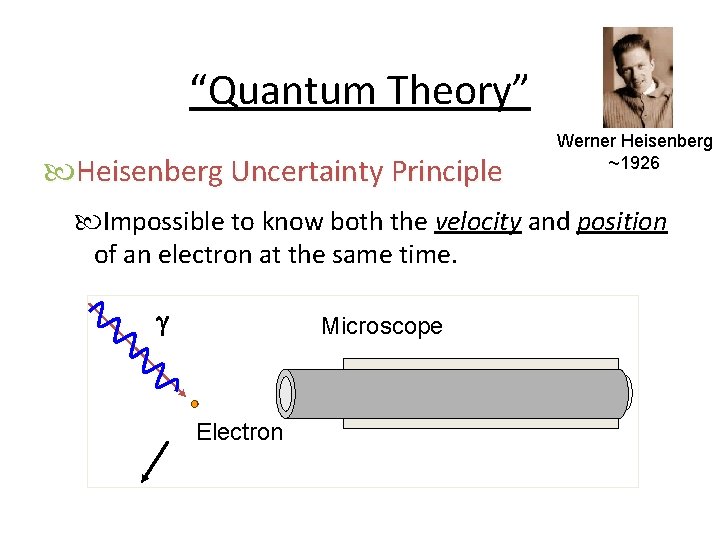
“Quantum Theory” Heisenberg Uncertainty Principle Werner Heisenberg ~1926 Impossible to know both the velocity and position of an electron at the same time. g Microscope Electron

“Quantum Theory” Pauli Exclusion Principle No more than two electrons in an atom can occupy the same orbital (may have zero, one, or two). Orbitals are defined as “s”, “p”, “d”, “f”…, each with different shapes. Each energy level has a specific types and number of orbitals in it. Electrons located in orbitals further away from nucleus contain greater energy.

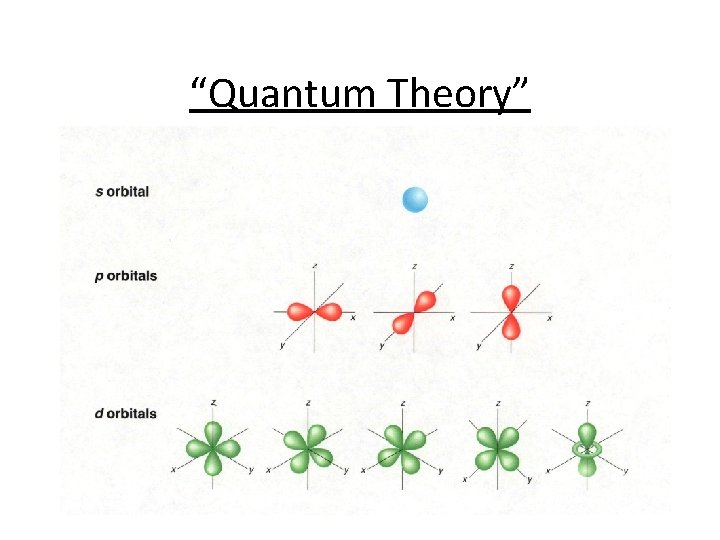
“Quantum Theory”

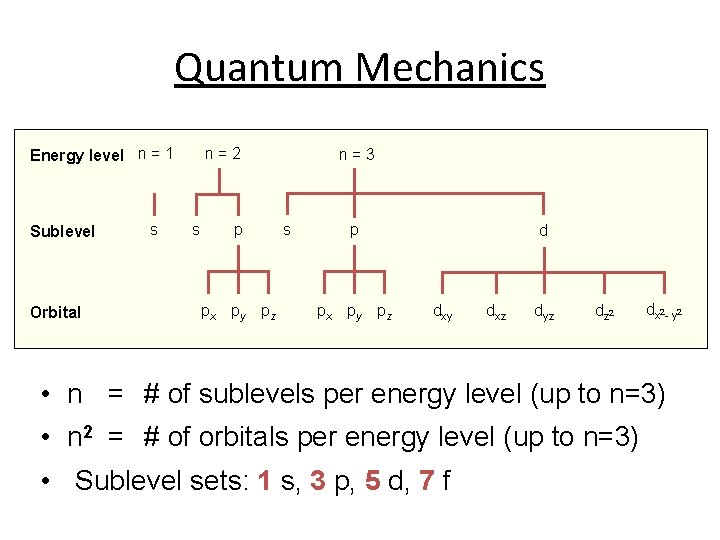
Quantum Mechanics Energy level n = 1 Sublevel Orbital s n=2 s p px py pz n=3 s p px py pz d dxy dxz dyz dz 2 dx 2 - y 2 • n = # of sublevels per energy level (up to n=3) • n 2 = # of orbitals per energy level (up to n=3) • Sublevel sets: 1 s, 3 p, 5 d, 7 f

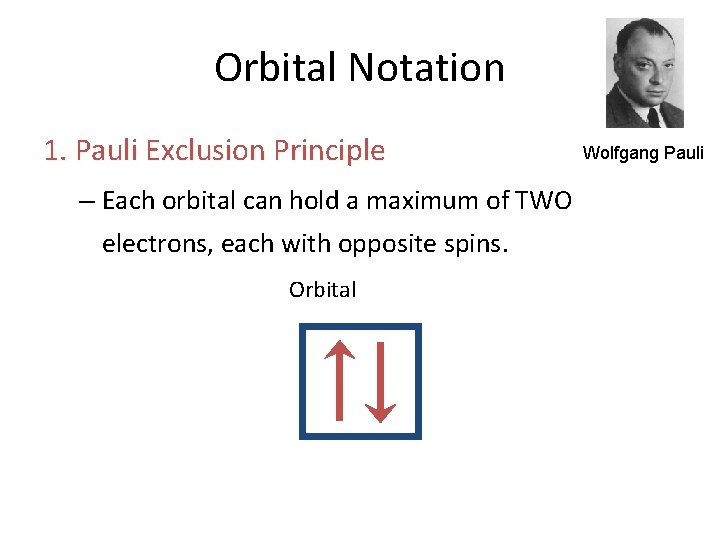
Orbital Notation 1. Pauli Exclusion Principle – Each orbital can hold a maximum of TWO electrons, each with opposite spins. Orbital Wolfgang Pauli

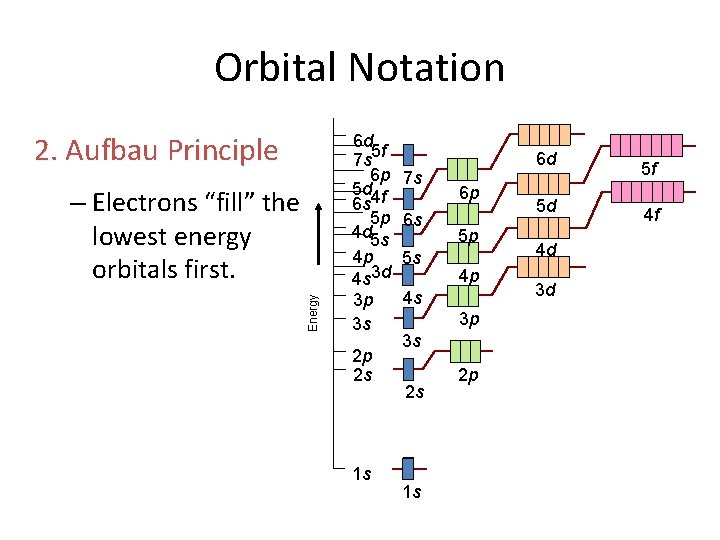
Orbital Notation 2. Aufbau Principle Energy – Electrons “fill” the lowest energy orbitals first. 6 d 7 s 5 f 6 p 5 d 4 f 6 s 5 p 4 d 5 s 4 p 4 s 3 d 3 p 3 s 2 p 2 s 1 s 7 s 6 s 5 s 6 d 6 p 5 p 4 p 4 s 3 p 3 s 2 s 1 s 2 p 5 d 4 d 3 d 5 f 4 f

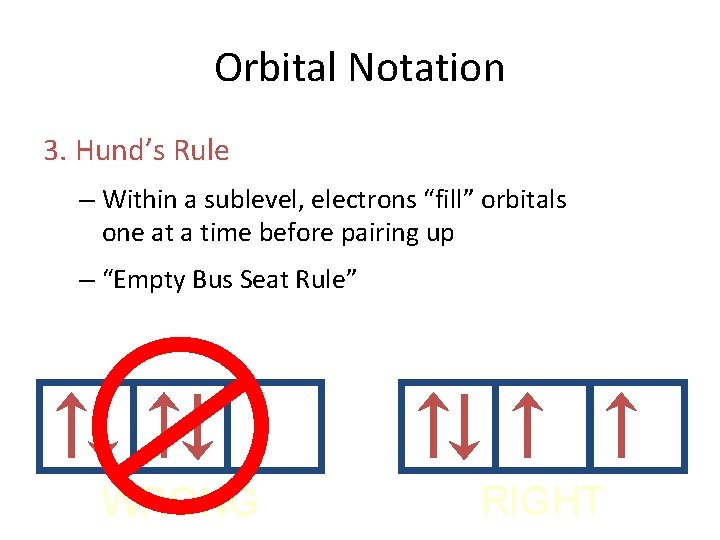
Orbital Notation 3. Hund’s Rule – Within a sublevel, electrons “fill” orbitals one at a time before pairing up – “Empty Bus Seat Rule” WRONG RIGHT

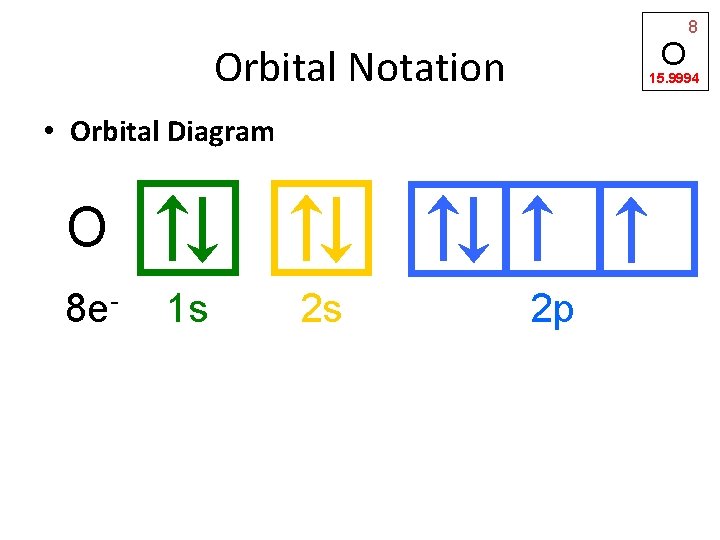
O Orbital Notation 15. 9994 • Orbital Diagram O 8 e- 1 s 2 s 8 2 p

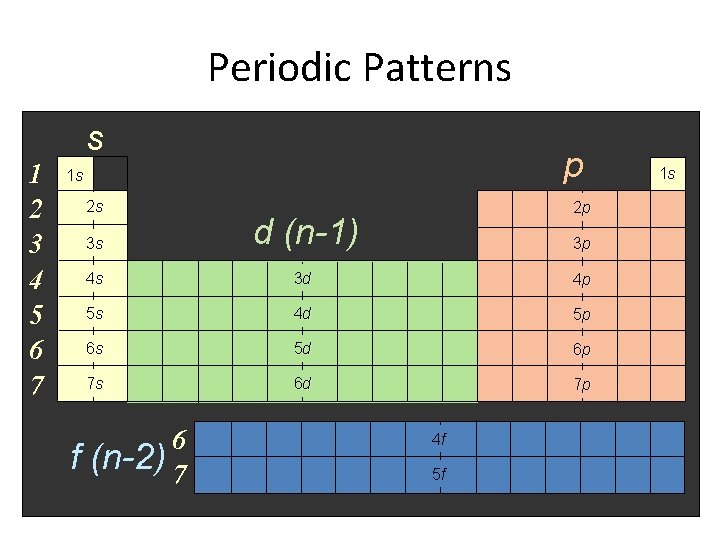
Periodic Patterns s 1 2 3 4 5 6 7 p 1 s 2 s f 2 p 3 s d (n-1) 4 s 3 d 4 p 5 s 4 d 5 p 6 s 5 d 6 p 7 s 6 d 7 p 6 (n-2) 7 3 p 4 f 5 f 1 s

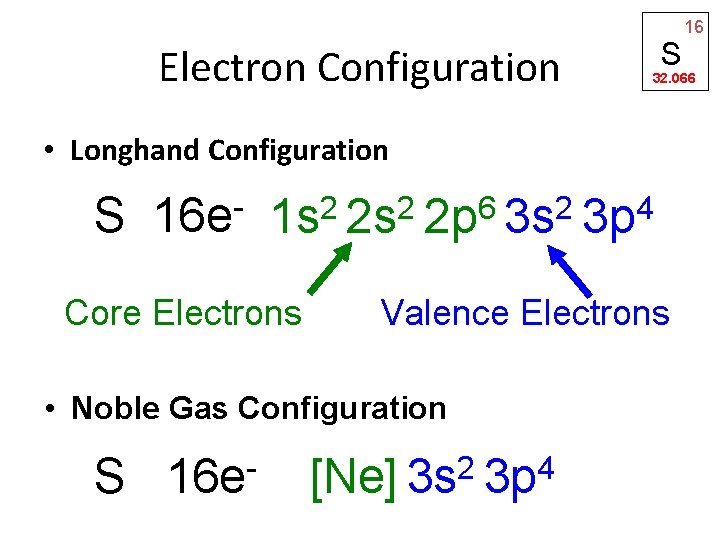
Electron Configuration S 32. 066 • Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons S 16 e 4 3 p Valence Electrons • Noble Gas Configuration 2 4 [Ne] 3 s 3 p 16

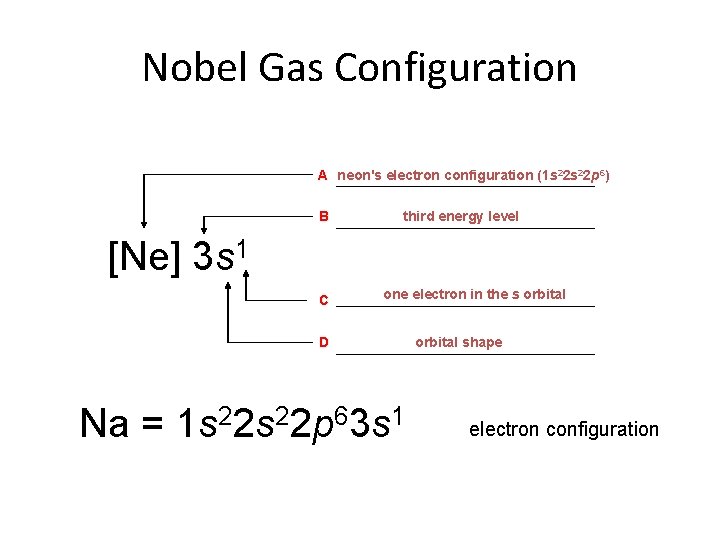
Nobel Gas Configuration A neon's electron configuration (1 s 22 p 6) B third energy level [Ne] 3 s 1 C one electron in the s orbital D Na = 1 s 22 p 63 s 1 orbital shape electron configuration

Stability • Ion Formation – Atoms gain or lose electrons to become more stable. – Isoelectronic with the Noble Gases.

Stability • Full energy level • Full sublevel (s, p, d, f) • Half-full sublevel

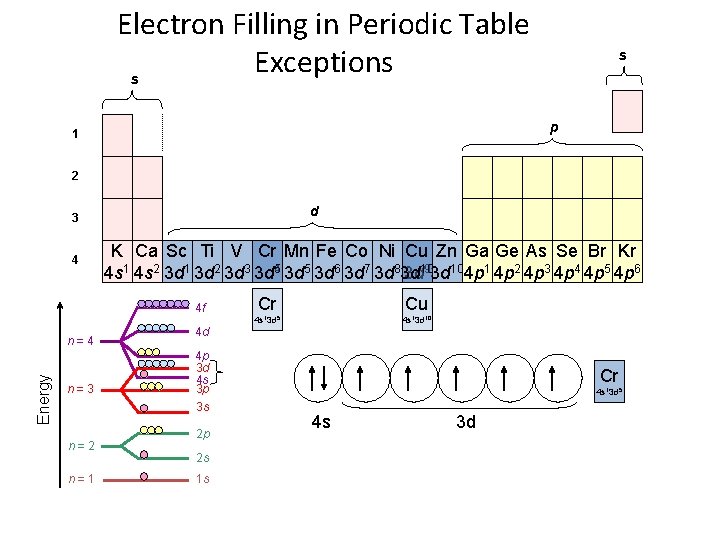
Electron Filling in Periodic Table Exceptions s s p 1 2 d 3 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 93 d 104 p 1 4 p 2 4 p 3 4 p 4 4 p 5 4 p 6 4 s 1 4 s 2 3 d 1 3 d 2 3 d 3 3 d 54 3 d 5 3 d 6 3 d 7 3 d 83 d 3 d 10 4 f Energy n=4 n=3 n=2 n=1 4 d 4 p 3 d 4 s 3 p 3 s 2 p Cr Cu 4 s 13 d 5 4 s 13 d 10 Cr 4 s 13 d 5 4 s 3 d 2 s Cu 1 s 4 s 13 d 10 4 s 3 d
![Stability Electron Configuration Exceptions Copper EXPECT Ar 4 s 2 3 d Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h/013696a914218dcf3e98e6017ba23ad0/image-21.jpg)
Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 – Copper gains stability with a full d-sublevel.
![Stability Electron Configuration Exceptions Chromium EXPECT Ar 4 s 2 3 d Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h/013696a914218dcf3e98e6017ba23ad0/image-22.jpg)
Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 – Chromium gains stability with a half-full d -sublevel.

Key Six Periodic Trends Atomic Radius: distance outermost electrons are from nucleus Ionization Energy: energy required to remove an electron from atom in gaseous state (endothermic) Metallic Character: metals lose e- to form cations; nonmetals gain e- to form anions Electronegativity: ability of an element to attract electrons within a covalent bond Electron Affinity: energy change when adding e(exothermic) Reactivity: ability of an element to react

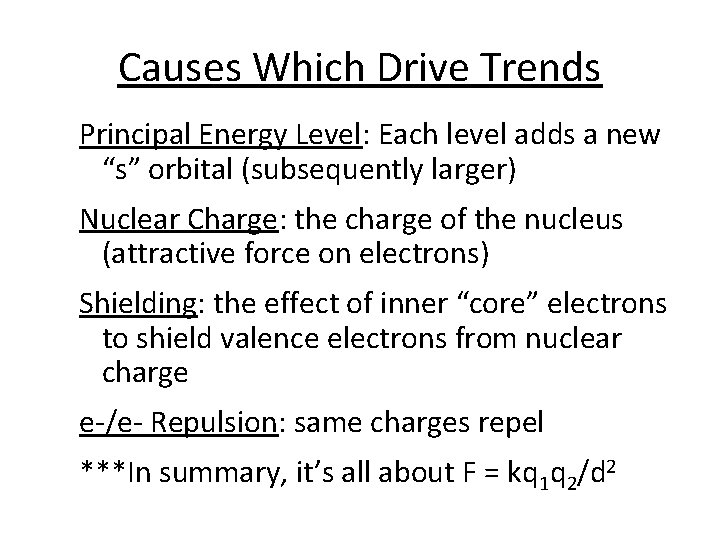
Causes Which Drive Trends Principal Energy Level: Each level adds a new “s” orbital (subsequently larger) Nuclear Charge: the charge of the nucleus (attractive force on electrons) Shielding: the effect of inner “core” electrons to shield valence electrons from nuclear charge e-/e- Repulsion: same charges repel ***In summary, it’s all about F = kq 1 q 2/d 2

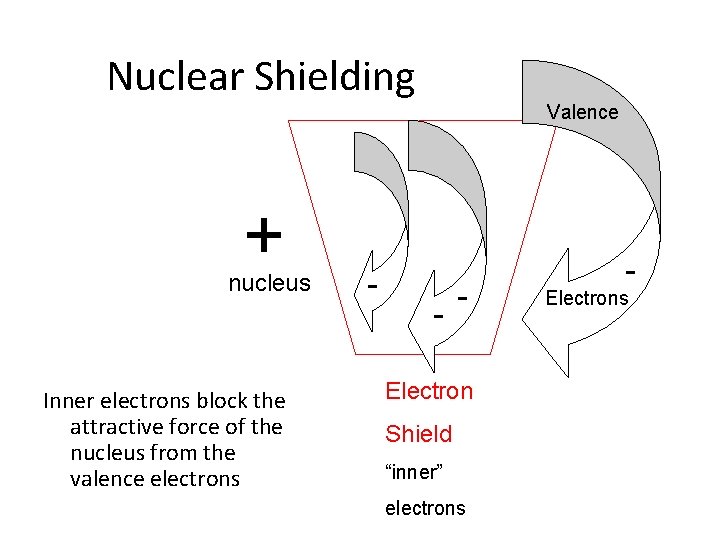
Nuclear Shielding + nucleus Inner electrons block the attractive force of the nucleus from the valence electrons - Valence - - Electron Shield “inner” electrons - Electrons

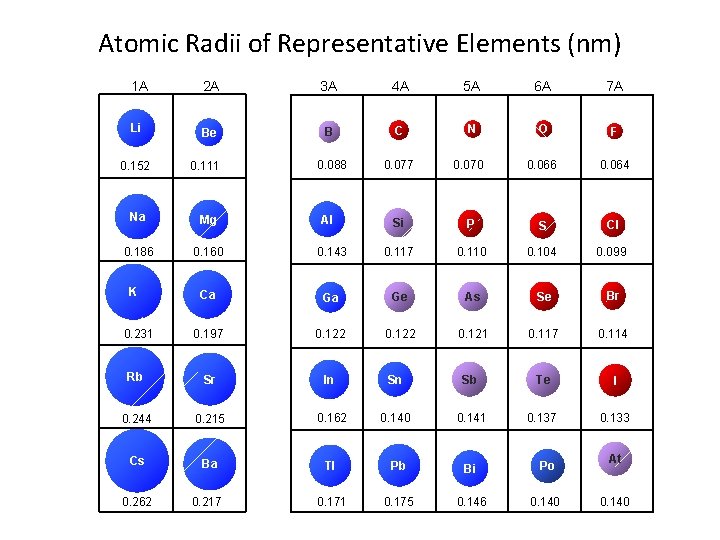
Atomic Radii of Representative Elements (nm) 1 A 2 A 3 A 4 A 5 A 6 A 7 A Li Be B C N O F 0. 088 0. 077 0. 070 0. 066 0. 064 Si P S Cl 0. 152 0. 111 Na Mg 0. 186 0. 160 0. 143 0. 117 0. 110 0. 104 0. 099 Ca Ga Ge As Se Br 0. 197 0. 122 0. 121 0. 117 0. 114 Rb Sr In Sn Sb Te I 0. 244 0. 215 0. 162 0. 140 0. 141 0. 137 0. 133 Cs Ba Tl Pb Bi Po 0. 262 0. 217 0. 171 0. 175 0. 146 0. 140 K 0. 231 Al At 0. 140

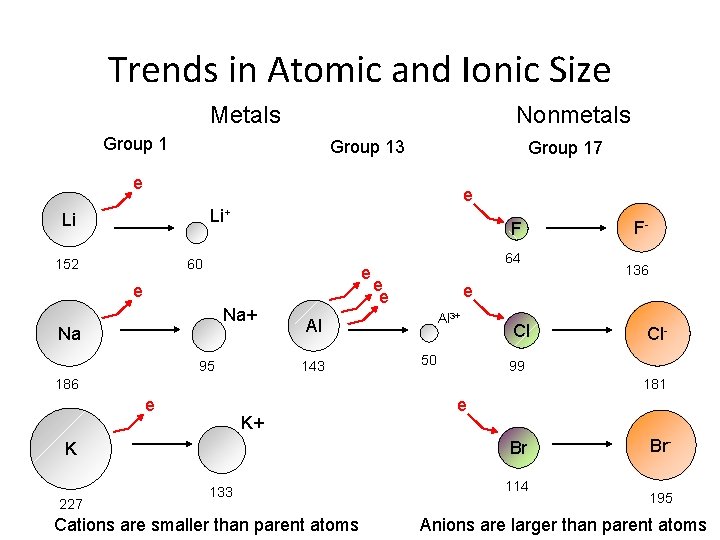
Trends in Atomic and Ionic Size Metals Nonmetals Group 13 Group 17 e e Li+ Li 152 F 60 e e Na+ Na 95 64 e e Al 3+ 50 Cl Cl- 99 186 181 e K+ e Br K 227 136 e Al 143 F- 133 Cations are smaller than parent atoms 114 Br 195 Anions are larger than parent atoms

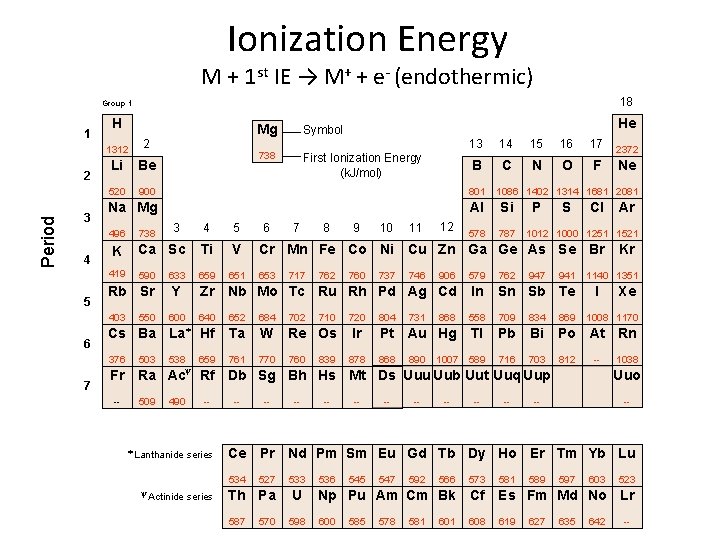
Ionization Energy M + 1 st IE → M+ + e- (endothermic) 18 Group 1 1 Period 2 3 H 6 7 738 First Ionization Energy (k. J/mol) 13 14 15 16 17 B C N O F 2 Li Be 520 900 801 Na Mg Al Si 578 787 12 Cl Ar 590 633 659 651 906 579 762 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 403 600 640 652 684 702 868 558 709 834 869 Cs Ba La* Hf Ta W Re Os Pt Au Hg Tl Pb Bi Po At Rn 376 538 659 761 770 760 868 589 716 703 812 Fr -- 503 11 S V 550 10 P Ti 7 9 1086 1402 1314 1681 2081 Ca Sc K 3 8 Ne 5 738 6 2372 4 419 5 Symbol 1312 496 4 He Mg 1012 1000 1251 1521 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 653 717 762 710 839 760 720 Ir 878 737 804 746 731 890 1007 941 1140 1351 I -- Ra Ac Rf Db Sg Bh Hs Mt Ds Uuu Uub Uut Uuq Uup 490 -- * Lanthanide series -- -- -- 1038 Uuo -- -- Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 534 y Actinide -- Xe 1008 1170 y 509 Kr 527 Th Pa 587 570 533 U 598 536 545 547 592 566 573 581 589 597 603 523 Np Pu Am Cm Bk Cf Es Fm Md No Lr 600 619 585 578 581 608 627 635 642 --

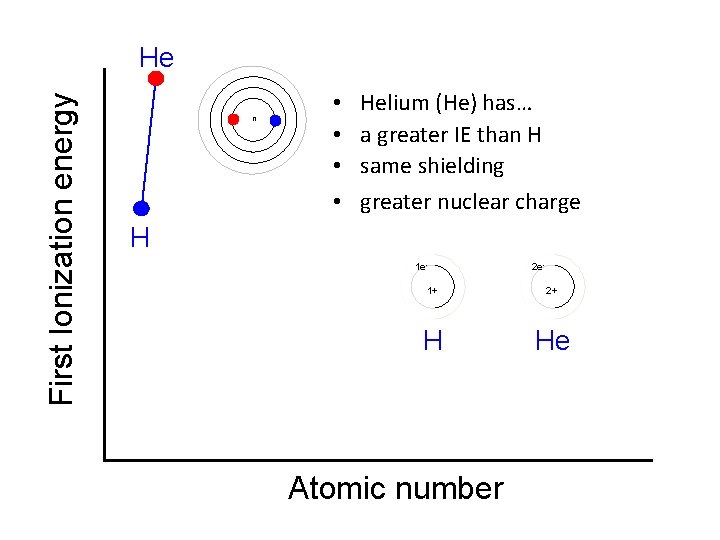
First Ionization energy He n • • Helium (He) has… a greater IE than H same shielding greater nuclear charge H 1 e- 2 e- 1+ 2+ H He Atomic number

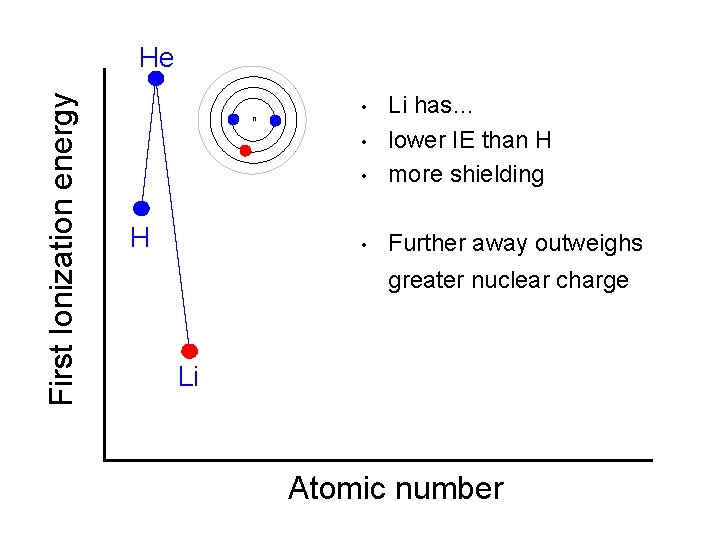
First Ionization energy He n • Li has… lower IE than H more shielding • Further away outweighs • • H greater nuclear charge Li Atomic number

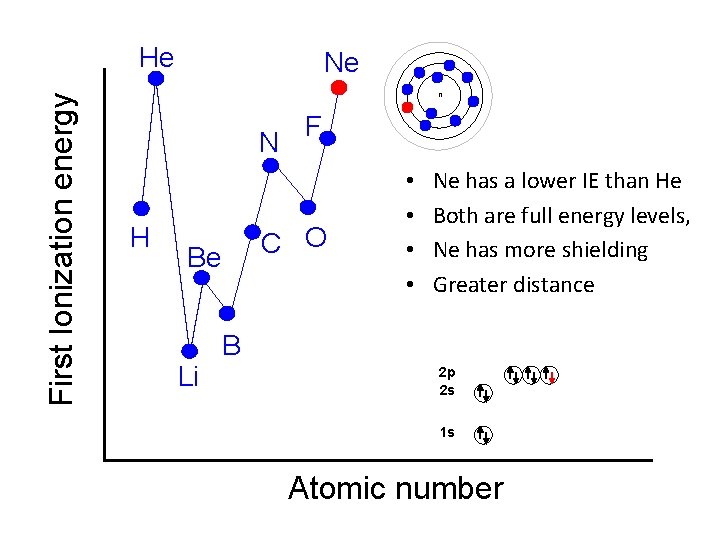
First Ionization energy He Ne n N H C O Be Li F • • Ne has a lower IE than He Both are full energy levels, Ne has more shielding Greater distance B 2 p 2 s 1 s Atomic number

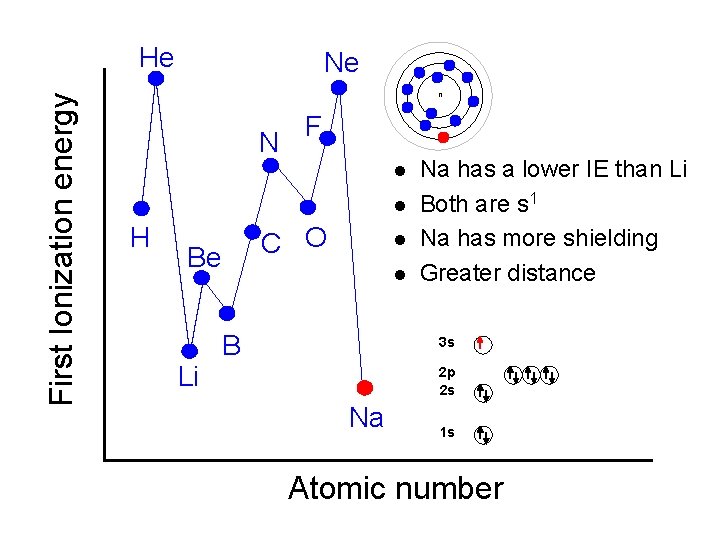
First Ionization energy He Ne n N F l l H C O Be Li l l B Na has a lower IE than Li Both are s 1 Na has more shielding Greater distance 3 s 2 p 2 s Na 1 s Atomic number

Metals, Nonmetals, & Metalloids 1 Nonmetals 2 3 4 5 Metals 6 7 Metalloids

Metallic Character metallic character increases nonmetallic character increases

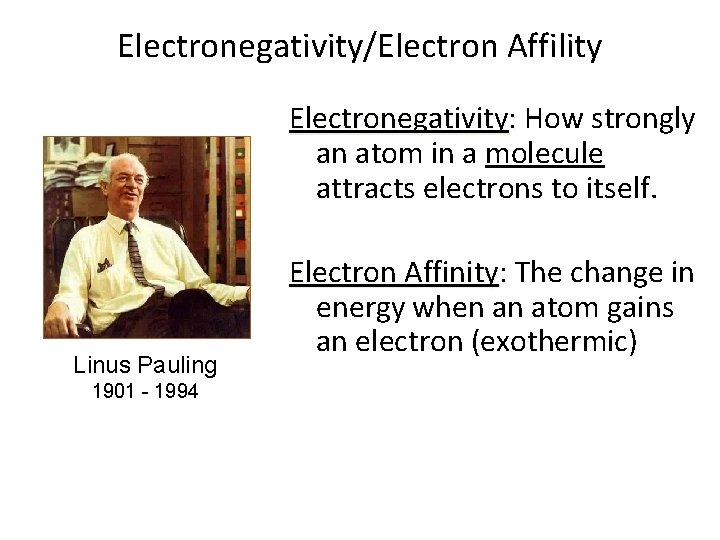
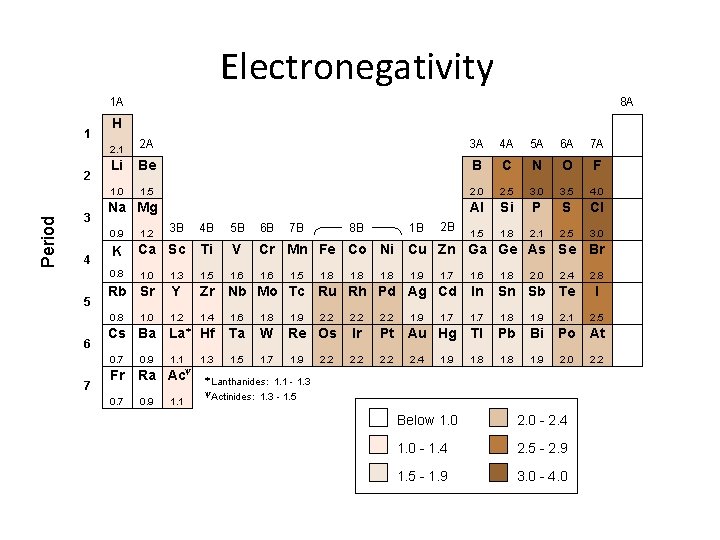
Electronegativity/Electron Affility Electronegativity: How strongly an atom in a molecule attracts electrons to itself. Linus Pauling 1901 - 1994 Electron Affinity: The change in energy when an atom gains an electron (exothermic)

Electronegativity 1 A 1 Period 2 3 4 5 6 7 8 A H 2. 1 2 A 3 A 4 A 5 A 6 A 7 A Li Be B C N O F 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 Al Si P S Cl 1. 5 1. 8 2. 1 2. 5 3. 0 Na Mg 1. 2 3 B 4 B 5 B 6 B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0. 8 1. 0 1. 3 1. 5 1. 6 1. 7 1. 6 1. 8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 0. 8 1. 2 1. 4 1. 6 1. 8 1. 9 2. 2 1. 7 1. 8 1. 9 2. 1 Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At 0. 7 1. 1 1. 3 1. 5 1. 7 1. 9 2. 2 1. 8 1. 9 2. 0 Fr 0. 7 1. 0 0. 9 y Ra Ac 0. 9 1. 1 8 B 7 B 1. 5 1. 8 2. 2 1. 8 1 B 2 B 0. 9 1. 8 1. 9 2. 4 1. 9 2. 0 2. 4 * Lanthanides: 1. 1 - 1. 3 - 1. 5 y. Actinides: Below 1. 0 2. 0 - 2. 4 1. 0 - 1. 4 2. 5 - 2. 9 1. 5 - 1. 9 3. 0 - 4. 0 2. 8 I 2. 5 2. 2

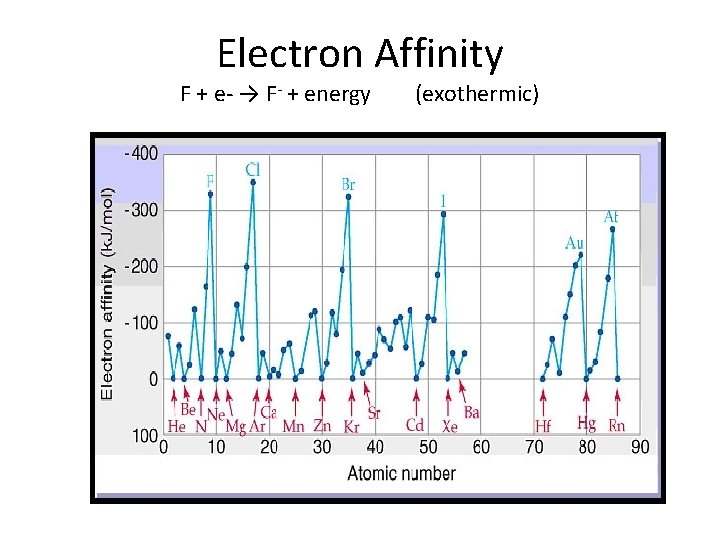
Electron Affinity F + e- → F- + energy (exothermic)

Reactivity GROUP 1 (ALKALI METALS) and 2 (ALKALINE-EARTH) Very reactive; become stable by losing 1 -2 electrons. Group 1 often stored in oil (most reactive group) GROUP 17 HALOGENS Most reactive of non-metals; become stable by gaining one electron GROUP 18 NOBLE GASES Low chemical reactivity; very stable with full energy level TRANSITION METALS Relatively unreactive

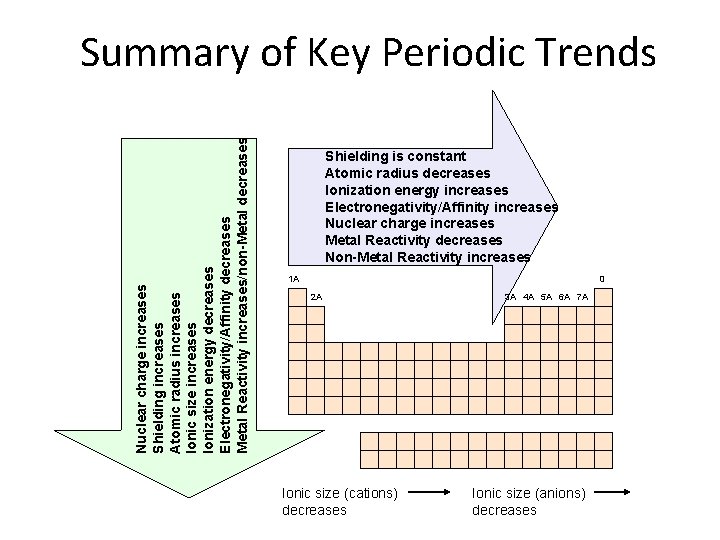
Nuclear charge increases Shielding increases Atomic radius increases Ionic size increases Ionization energy decreases Electronegativity/Affinity decreases Metal Reactivity increases/non-Metal decreases Summary of Key Periodic Trends Shielding is constant Atomic radius decreases Ionization energy increases Electronegativity/Affinity increases Nuclear charge increases Metal Reactivity decreases Non-Metal Reactivity increases 1 A 0 2 A Ionic size (cations) decreases 3 A 4 A 5 A 6 A 7 A Ionic size (anions) decreases