Models for Implementing Artificial Intelligence in Pathology Practice






























- Slides: 48

Models for Implementing Artificial Intelligence in Pathology Practice May 6, 2019 Douglas J. Hartman, MD

Disclosures �Philips = honorarium for educational presentation

Objectives 1. Describe an evolving model for the diagnostic cockpit and how that applies to pathology 2. Demonstrate a sample diagnostic signout workflow 3. Describe how artificial intelligence can be integrated into the pathology diagnostic workflow

Pathology Cockpit �Early discussions of a pathology cockpit in 2010 �Very little literature about this exists Diagn Pathol. 2014; 9 Suppl 1: S 12. doi: 10. 1186/1746 -1596 -9 -S 12. Epub 2014 Dec 19. i. Pathology cockpit diagnostic station: validation according to College of American Pathologists Pathology and Laboratory Quality Center recommendation at the Hospital Trust and University of Verona. Brunelli M, Beccari S, Colombari R, Gobbo S, Giobelli L, Pellegrini A, Chilosi M, Lunardi M, Martignoni G, Scarpa A, Eccher A.

Cockpit Evolution � Forum sponsored by the Academy for Radiology and Biomedical Imaging Research � Brought together stakeholders from: � Urology � Oncology � Pathology � Neurology � Cardiology � Emergency Medicine � Molecular Diagnostics � Informatics

Goals of the Symposium 1. 2. 3. 4. 5. Elevate the profile of medical imaging technologies Ensures its value and impact are broadly recognized Facilitate collaborations Highlight content experts Provide critical voice of the imaging community

Drivers for convening symposium �Errors and imprecision in medical diagnosis �Leads to poor patient outcomes �Two common diagnostic errors � Ordering the wrong imaging test � Misinterpretation of imaging test findings

Cockpit = “Integrated Diagnostics” �Current Obstacles: �Limited dissemination of valuable diagnostic technologies �Siloed electronic health records �Few available data analytic tools �Variable data inputs and outputs �Lack of coordinated effort to improve diagnostics across all stakeholders

Symposium Composition Presentations from key stakeholders (“consumers”) 2. Presentations from the “Cockpit Crew” (users) 3. Multidisciplinary groups to identify models and prioritize the next steps in constructing a prototype Cockpit 1.

High Priority Tasks � Develop national standards � Catalog available data analytic methods � Develop advanced analytic methods � Develop environment to host the analytic methods � Create an environment to enable and facilitate communication/cooperation � Catalyze the construction of the prototype Cockpit

Advancing the Diagnostic Cockpit �May 2018 �Four objectives: � Standardization/interoperab ility � Application of advanced computation � Acceleration of development and translation of new techniques � Promotion of best practices in medical imaging

Challenges �Lack of standardized measurement and techniques �Lack of standard interchange mechanism �Need for more standardized reference studies

Action Items from Symposium 1. 2. 3. 4. 5. Identify neutral third party to validate de-identification, manage datasets and ensure interoperability Collect 100 complete datasets from 10 different institutions Create compendium of existing standards Refine core functional requirements of the diagnostic cockpit Identify potential funding sources for any initiative

�So what does this mean for pathology?

Digital Pathology Background �Very few labs in the USA have gone entirely digital for primary diagnosis �UPMC is restarting their conversion to a digital pathology platform for primary diagnosis sign out �Vendors have been regulated by Food Drug Administration – WSI considered Class III device �First FDA approved system – Philips – April 2017

UPMC Clinical Use Cases �Retrospective Slide Scanning (passive encouragement of slide scanning – archival use case) �Internal (UPMC) & external consults �IHC core lab (centralization) �Breast marker image analysis �Philips Tutor (formerly Path. XL) education partnership �To do: �Archive outside consults, frozen section, tumor board, etc.

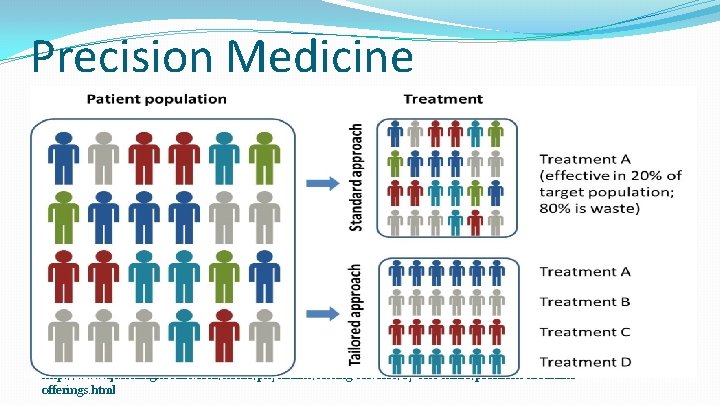
Precision Medicine http: //www. questdiagnostics. com/home/physicians/testing-services/by-test-name/precision-medicineofferings. html

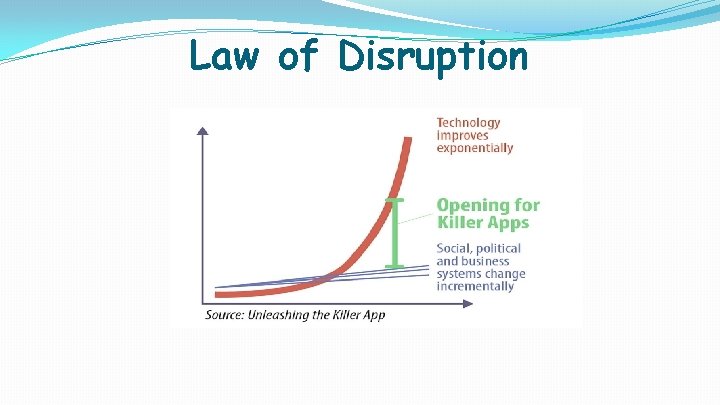
Law of Disruption

The Perfect Storm “When technologies, products, and services converge in radical, creative new ways, a killer app can emerge” Services Products Technology Dones & Mui. Unleashing the killer app. Harvard Business School Press. 2000

Algorithms • Identify rare events (e. g. screening for microorganisms) • Quantitative measurements • Score biomarkers (e. g. ER, PR, Her 2/neu, Ki 67, CD 34, PD-L 1) • Tissue measurements (e. g. mitotic counts, quantify fibrosis/steatosis) • Analyze spatial patterns and feature distribution (e. g. neuroscience) • Automated grading (of tumors) • CAD (e. g. prostate cancer diagnosis, detect Barrett’s esophagus with dysplasia) • Workflow (smart) algorithm (e. g. triage cases, automate downstream steps like LCM) • Miscellaneous (research & novel) algorithms (e. g. TMAs, 3 D image reconstruction)



Integration in Digital Signout -1

Integration in Digital Signout -2

Integration in Digital Signout -3

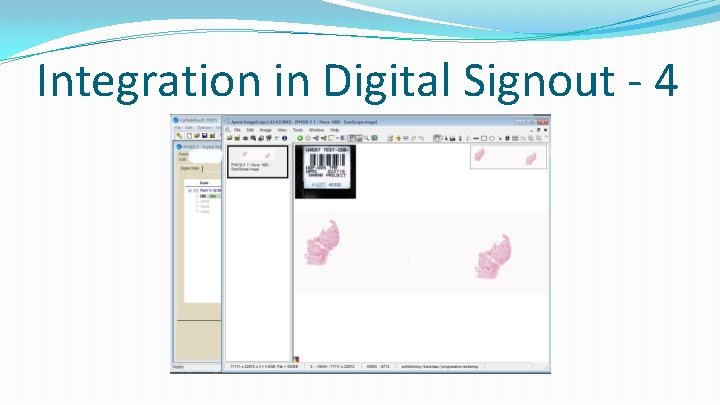
Integration in Digital Signout - 4

Digital Workflow Legacy Workflow Digital Workflow

Integration in Digital Signout - 5

Integration in Digital Signout -6

Implemented Image Analysis �Automated CD 8 quantification �Digital tumor bud assessment �Automated Her 2 assessment in GE junction tumors �Developing more biomarker evaluation

CD 8 Analysis Oropharyngeal Cases �? Selection criteria to use �Tonsillar tissue �Resection vs biopsy vs TMA core

Whole section Analysis

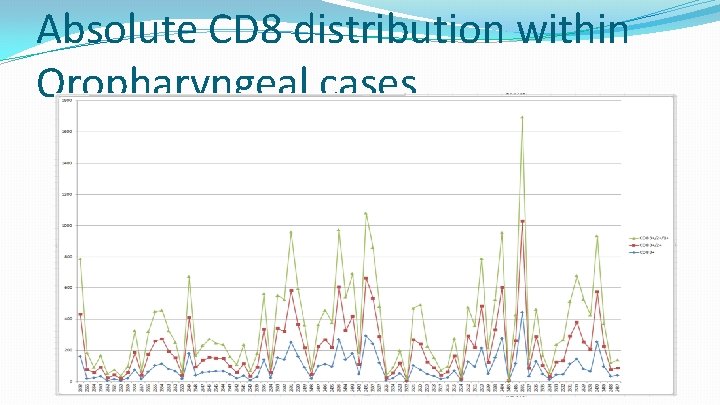
Absolute CD 8 distribution within Oropharyngeal cases

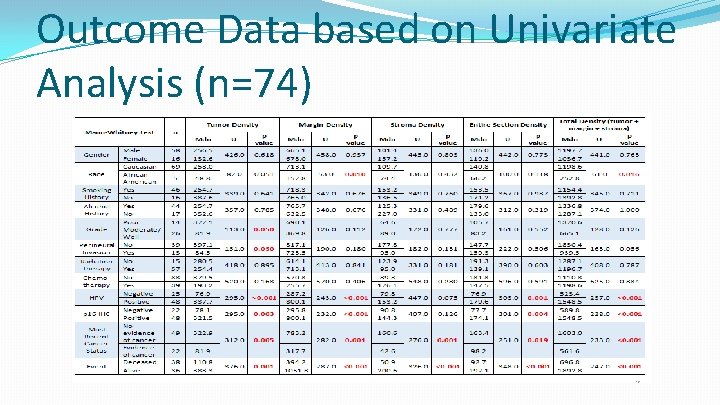
Outcome Data based on Univariate Analysis (n=74)

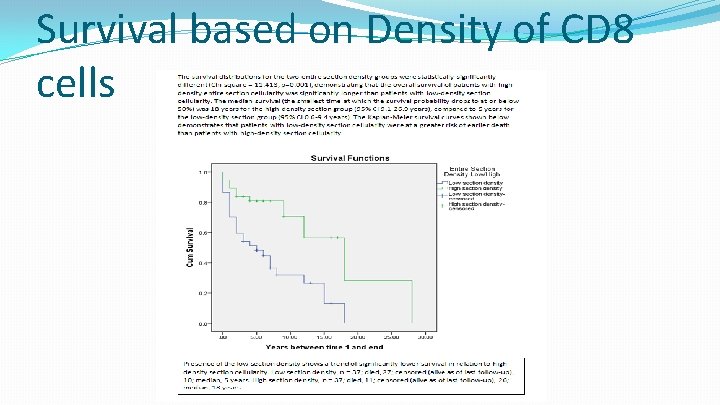
Survival based on Density of CD 8 cells

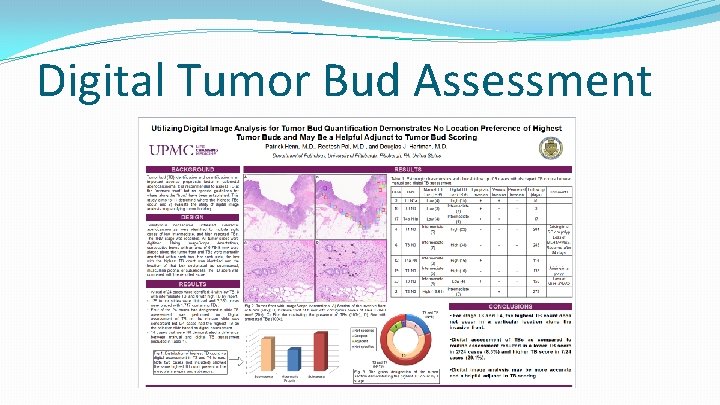
Digital Tumor Bud Assessment

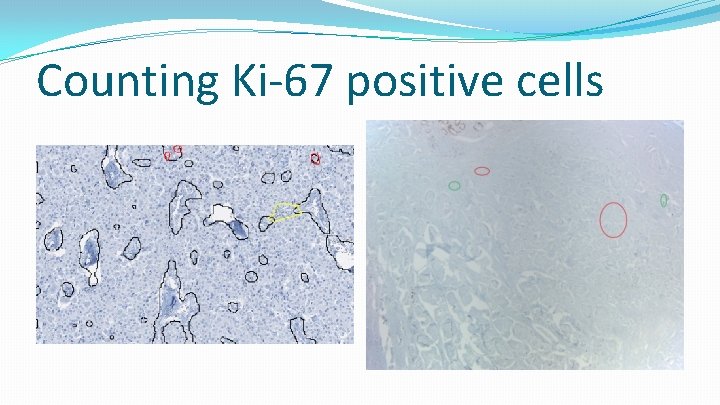
Counting Ki-67 positive cells

Regulatory �Few AI-based algorithms have been cleared by the Food and Drug Administration �Use cases: �Bone fracture �Large vessel occlusion �Brain damage �Diabetic retinopathy

Payment No billing code specifically associated with artificial intelligence 2. Options: 1. 2. 3. Per Click Flat fee Other?


Public Image Challenges �Image analysis challenges �Camelyon – 16 – detecting metastases �Cataloged at Grand Challenges �Public leaderboard of contestants

Summary of Pathology Image Challenges � 19/169 challenges involved pathology � 15 -1000 slides �Ranged from 10 -40 x magnification �The number of recorded participants ranged from 13 -1231

Summary of Pathology Image Challenges – organ area and file types �Organ sites: �Breast (9) �Cervix (2) �Neuropath (2) �Multiorgan (2) �Thyroid (1); Colorectal (1); lung (1); hemepath (1) �Most common to have a single file type (15 challenges)

Image Challenges - evaluation � Various statistical methods � Dice Coefficient � Area under the curve � Weighted precision � Free response operating curve � F 1 score � Sensitivity/specificity � Gold standard � One pathologist � Multiple “consensus” pathologists � Medical Experts � Oncologist � Not mentioned

Future Directions �Cockpit for Pathology already forming (mostly around software system integration) �Most designs have tried to emulate current workflows �Novel interactions with whole slide images (Virtual reality/augmented reality)

Conclusions �Artificial Intelligence based tools are ready for use in the diagnostic pathology workflow �Building interoperability with digital pathology systems is critical to adoption �Integrating artificial intelligence into digital pathology platforms with increase adoption and facilitate more utilization of this powerful technology

Questions and Answers Douglas J. Hartman MD hartmandj@upmc. edu

References 1. 2. 3. 4. 5. 6. Mark D. Zarella, Douglas Bowman; , Famke Aeffner, Navid Farahani, Albert Xthona; , Syeda Fatima Absar, Anil Parwani, Marilyn Bui, and Douglas J. Hartman (2019) A Practical Guide to Whole Slide Imaging: A White Paper From the Digital Pathology Association. Archives of Pathology & Laboratory Medicine: February 2019, Vol. 143, No. 2, pp. 222 -234. Guo H, Birsa J, Farahani N, Hartman DJ, Piccoli A, O'Leary M, Mc. Hugh J, Nyman M, Stratman C, Kvarnstrom V, Yousem S, Pantanowitz L. Digital pathology and anatomic pathology laboratory information system integration to support digital pathology sign-out. J Pathol Inform. 2016 May 4; 7: 23. doi: 10. 4103/2153 -3539. 181767. e. Collection 2016. PMID: 27217973 http: //www. acadrad. org/wp-content/uploads/2019/01/Academy-White-Paper-Sympoiusm-to. Address-Standards-2018. pdf http: //www. questdiagnostics. com/home/physicians/testing-services/by-test-name/precisionmedicine-offerings. html https: //grand-challenge. org/challenges/ Ratner M. FDA backs clinician-free AI imaging diagnostic tools. Nat Biotechnol 2018 Aug 6; 36(8): 673 -674.