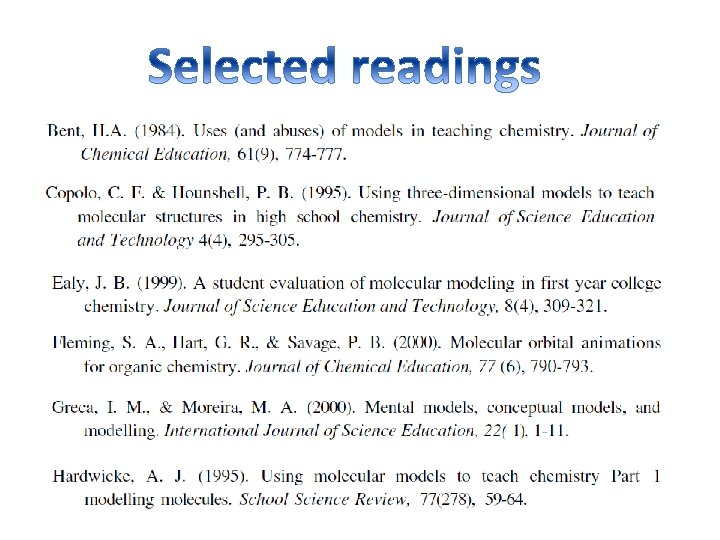
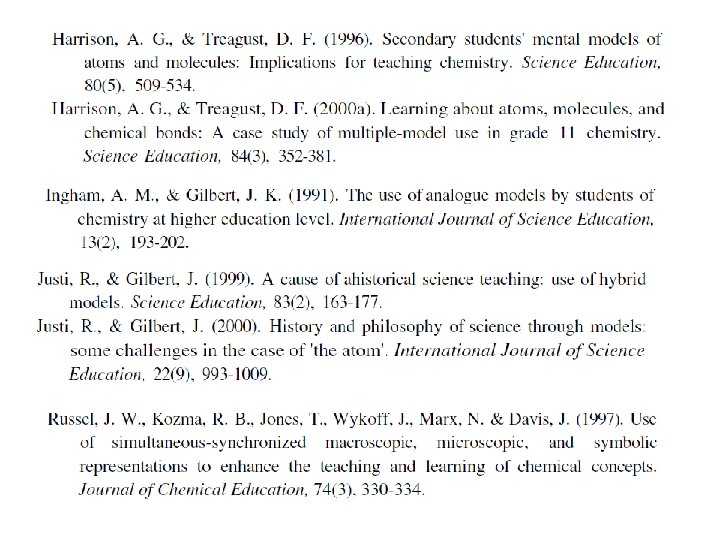
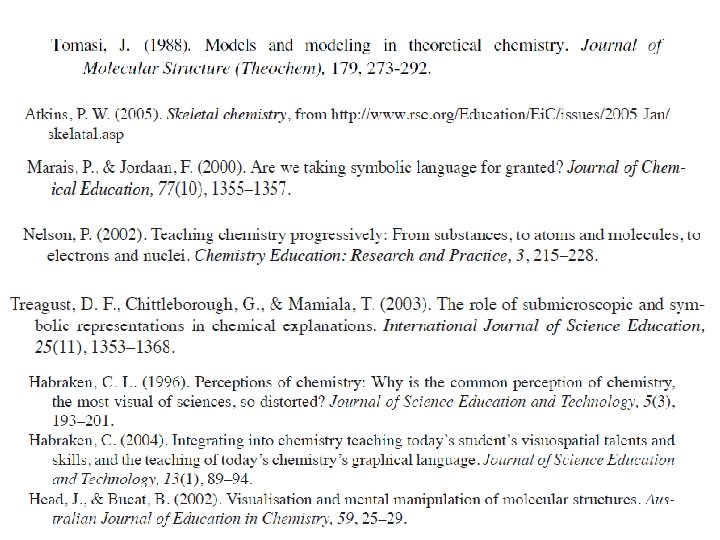
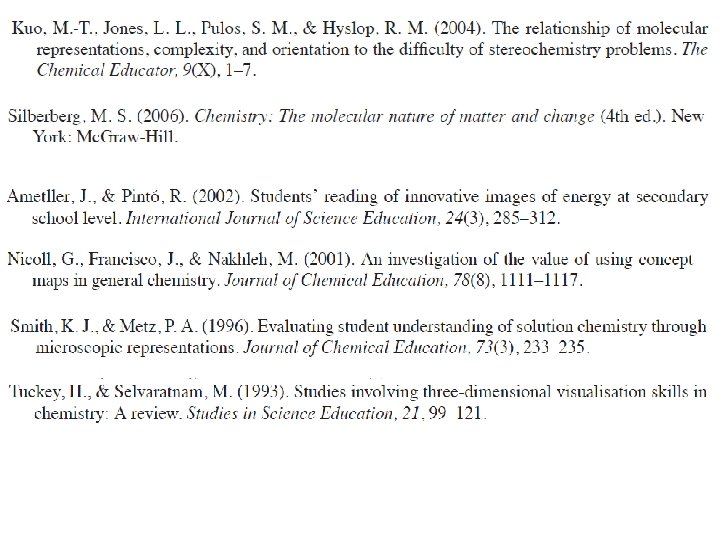
Models and Modelling in Chemical Educations Chemists model





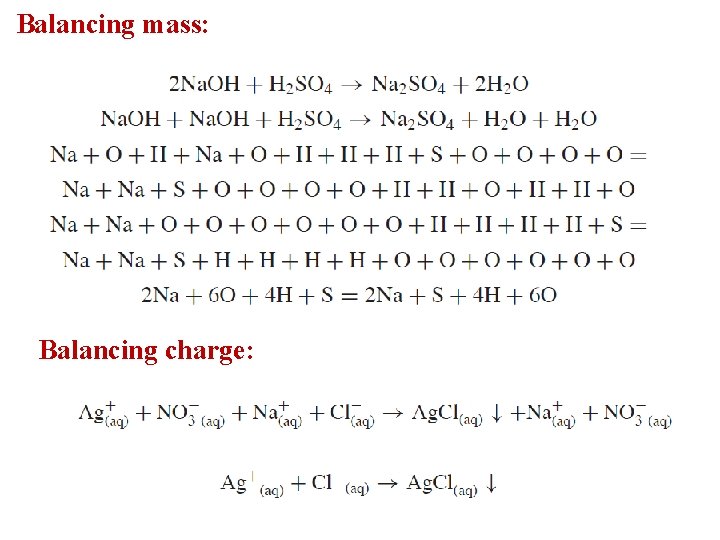


- Slides: 47

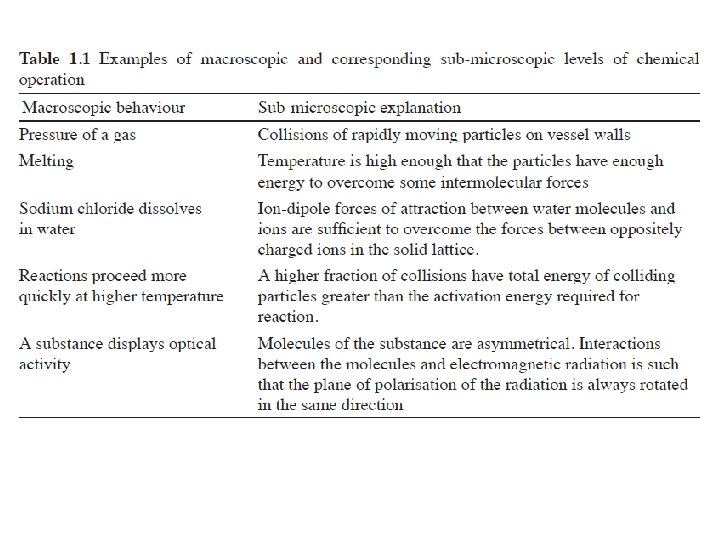
Models and Modelling in Chemical Educations Chemists model both the phenomena they observe (macro level) and the ideas with which they try to explain such phenomena (sub -micro level) by the use of analogy with what they already know. Symbolism, chemical language ‘Modelling is so common in chemistry that it has become the dominant way of thinking’

Explanations of the natures of substances and of their transformations are essentially abstract. Molecular models thus became obligatory tools in the study of stereochemistry, properties and reactivity of substances, which, in turns, corroborated the atomic theory. - Computational models - Quantum chemistry-based models

Triplet representation of Chemistry Macro: Phenomena Sub-micro: Interpretation, Model, Explanation Symbolic level

Macro: p. H, T, mass, density, concentration, osmotic pressure…. Sub-micro: the particulate nature of Matter Symbolic: chemical equations (quantitative assessment)


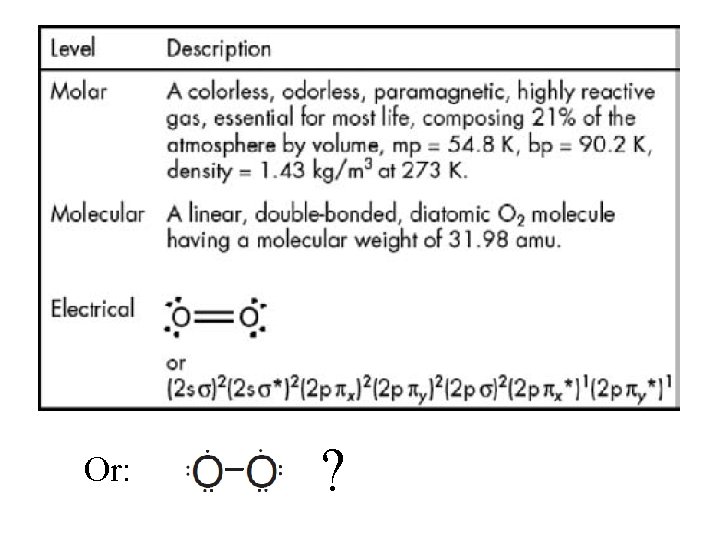
Or: ?

Issue: What does it mean that electrons in the 3 p obital of an atom can interpenetrate the distributions of those electrons in the 1 s, 2 s and 2 p orbitals?

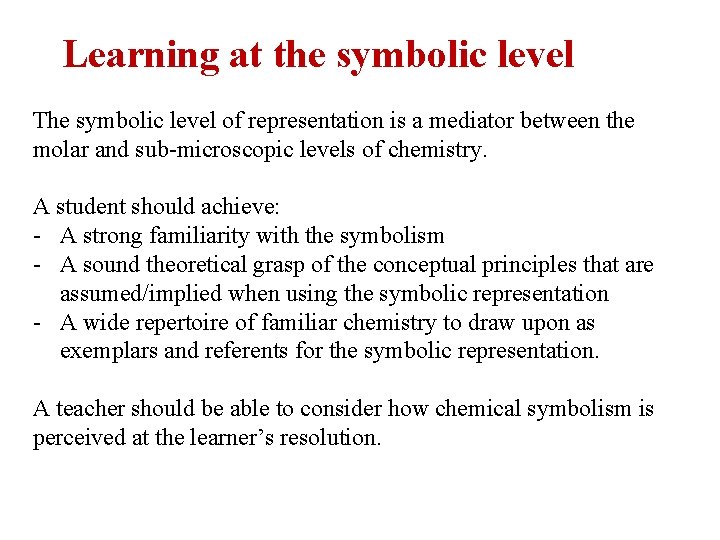
Learning at the symbolic level The symbolic level of representation is a mediator between the molar and sub-microscopic levels of chemistry. A student should achieve: - A strong familiarity with the symbolism - A sound theoretical grasp of the conceptual principles that are assumed/implied when using the symbolic representation - A wide repertoire of familiar chemistry to draw upon as exemplars and referents for the symbolic representation. A teacher should be able to consider how chemical symbolism is perceived at the learner’s resolution.

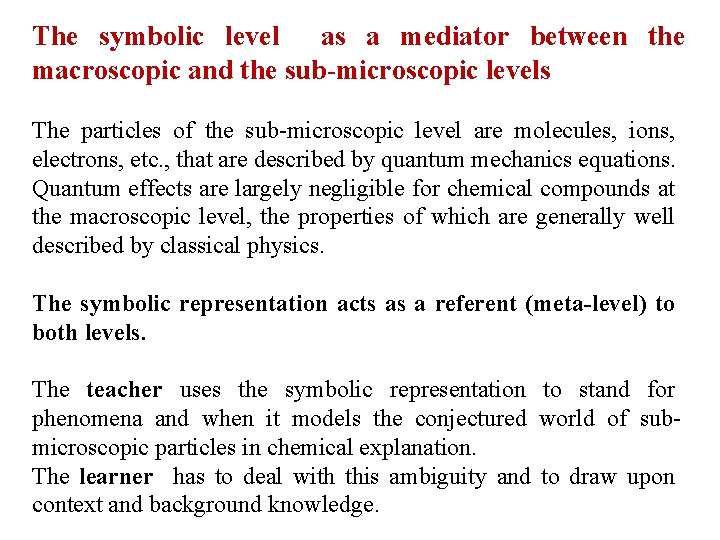
The symbolic level as a mediator between the macroscopic and the sub-microscopic levels The particles of the sub-microscopic level are molecules, ions, electrons, etc. , that are described by quantum mechanics equations. Quantum effects are largely negligible for chemical compounds at the macroscopic level, the properties of which are generally well described by classical physics. The symbolic representation acts as a referent (meta-level) to both levels. The teacher uses the symbolic representation to stand for phenomena and when it models the conjectured world of submicroscopic particles in chemical explanation. The learner has to deal with this ambiguity and to draw upon context and background knowledge.


Chemical language Symbols are not just labels for words, but closely linked to concepts.

Learning chemical language A student should: - Learn the allowed symbols and what they represent - Understand the grammar of the representational language - Know enough chemistry to be bale to compose ‘true’ statements using the chemical language.

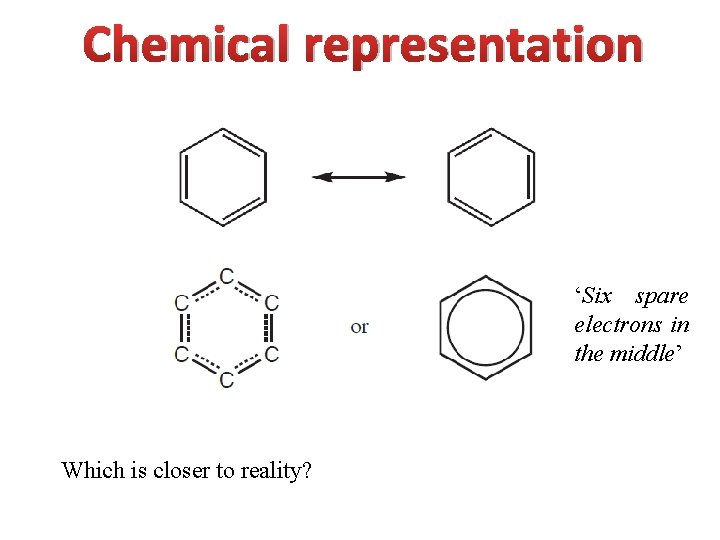
Chemical representation ‘Six spare electrons in the middle’ Which is closer to reality?

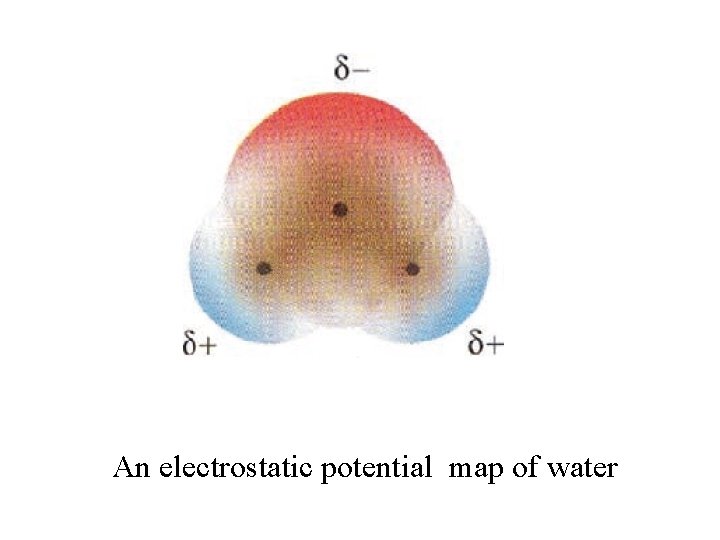
An electrostatic potential map of water

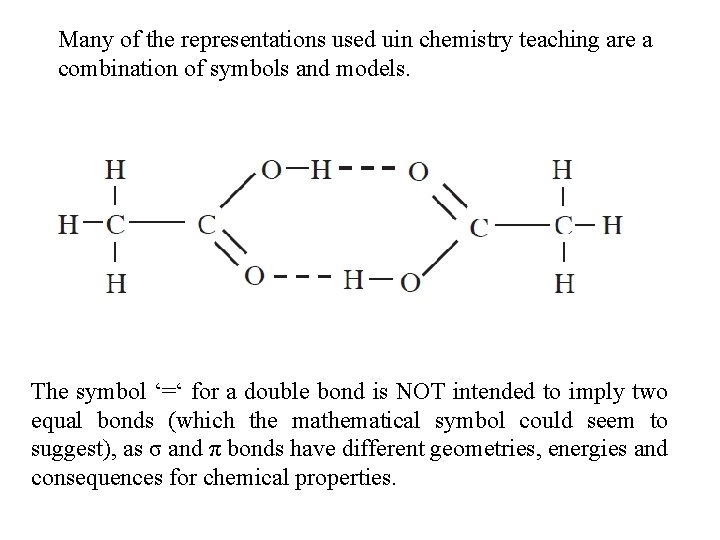
Many of the representations used uin chemistry teaching are a combination of symbols and models. The symbol ‘=‘ for a double bond is NOT intended to imply two equal bonds (which the mathematical symbol could seem to suggest), as σ and π bonds have different geometries, energies and consequences for chemical properties.

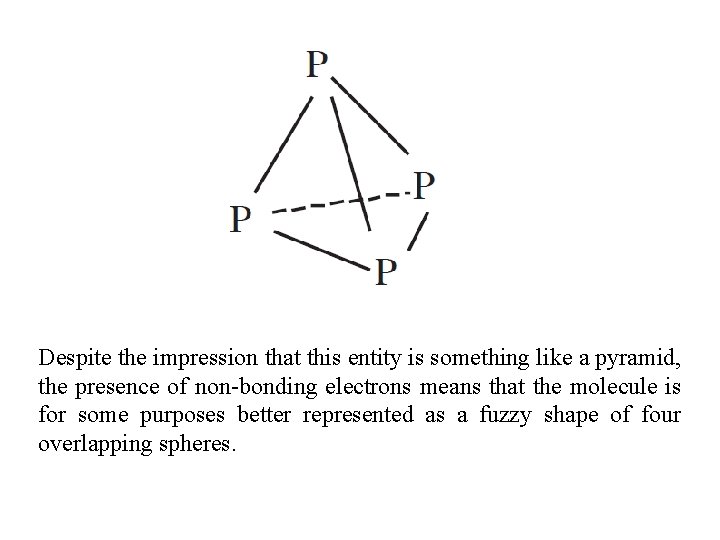
Despite the impression that this entity is something like a pyramid, the presence of non-bonding electrons means that the molecule is for some purposes better represented as a fuzzy shape of four overlapping spheres.

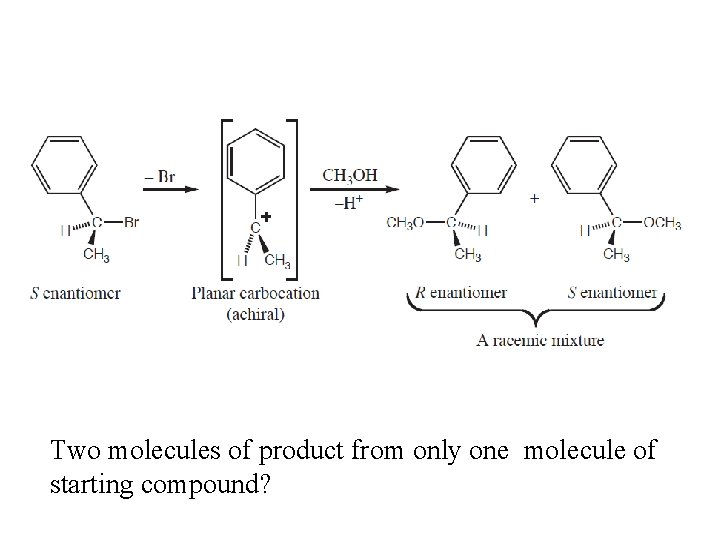
Two molecules of product from only one molecule of starting compound?

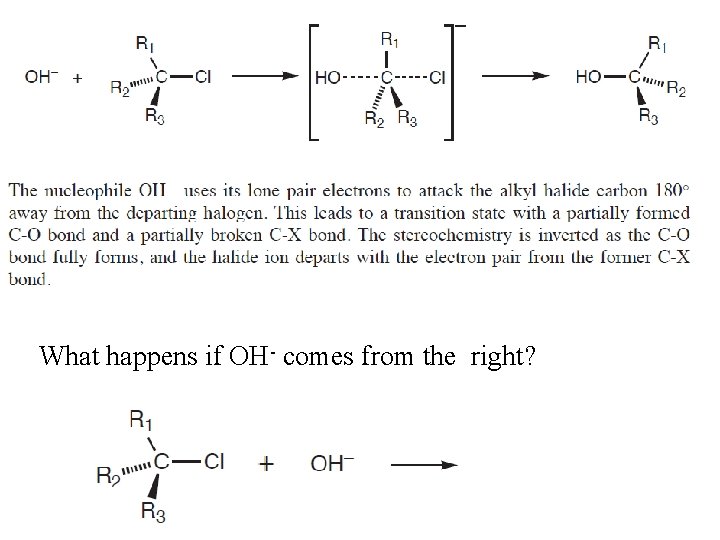
What happens if OH- comes from the right?

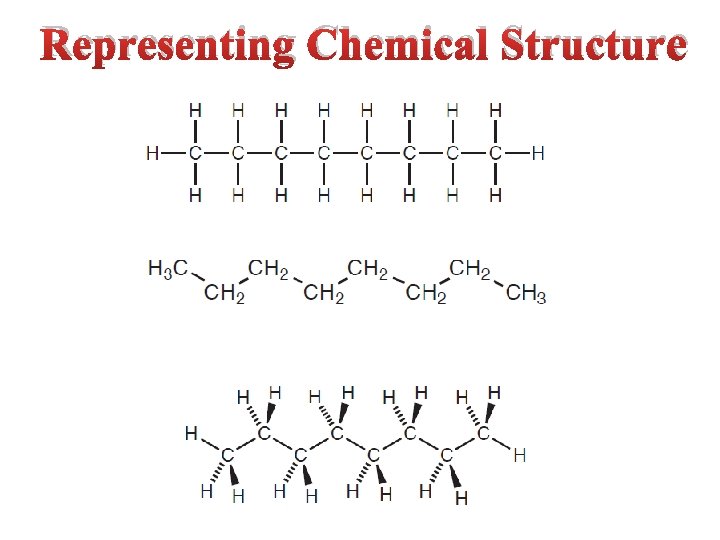
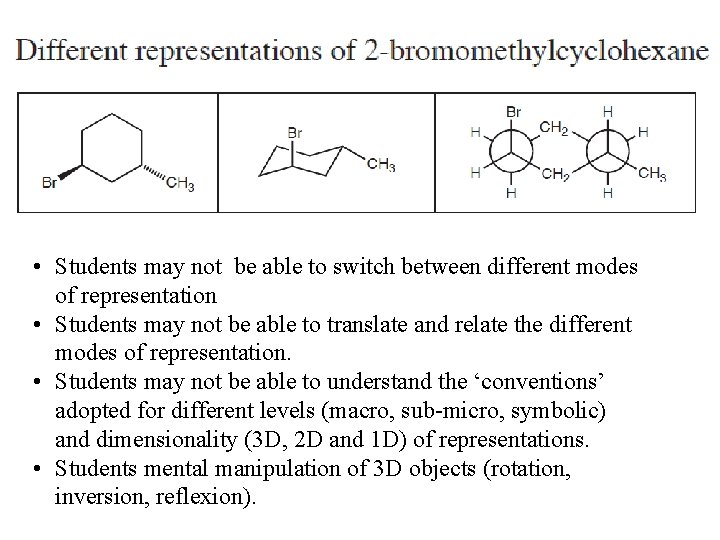
Representing Chemical Structure

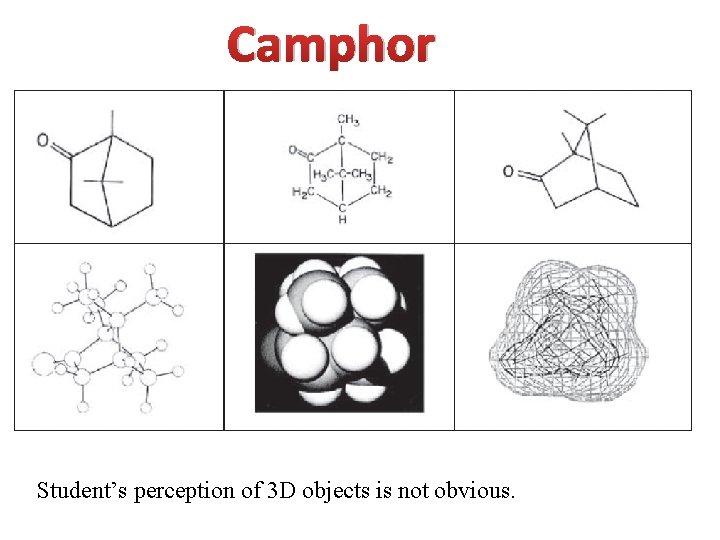
Camphor Student’s perception of 3 D objects is not obvious.

• Students may not be able to switch between different modes of representation • Students may not be able to translate and relate the different modes of representation. • Students may not be able to understand the ‘conventions’ adopted for different levels (macro, sub-micro, symbolic) and dimensionality (3 D, 2 D and 1 D) of representations. • Students mental manipulation of 3 D objects (rotation, inversion, reflexion).

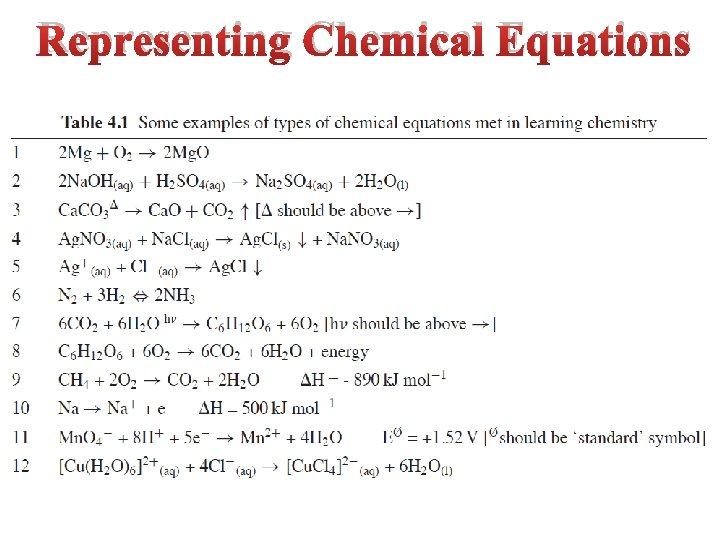
Representing Chemical Equations

Learning the basic grammar of chemical equations ‘A key feature of a chemical equation is that it has two parts, representing ‘before’ anfd ‘after’ the process. By convention, the left hand side of the equation represents before the process, and the right hand side represents what is present afterwards’. Issue: Do you agree with this statement?

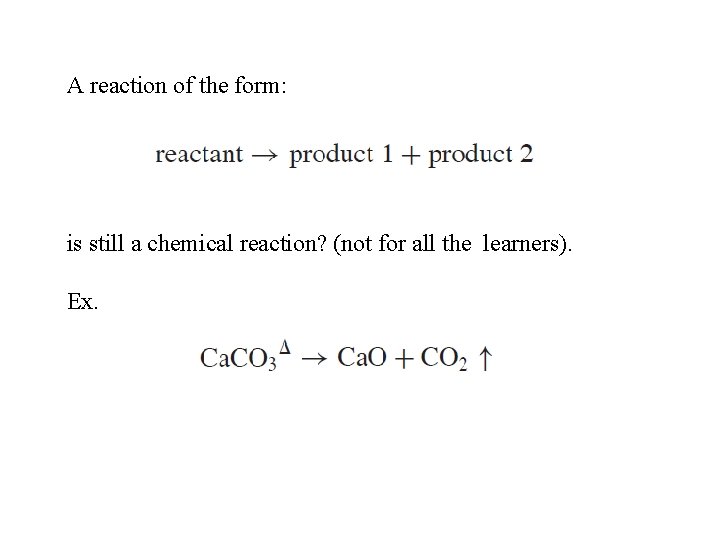
A reaction of the form: is still a chemical reaction? (not for all the learners). Ex.
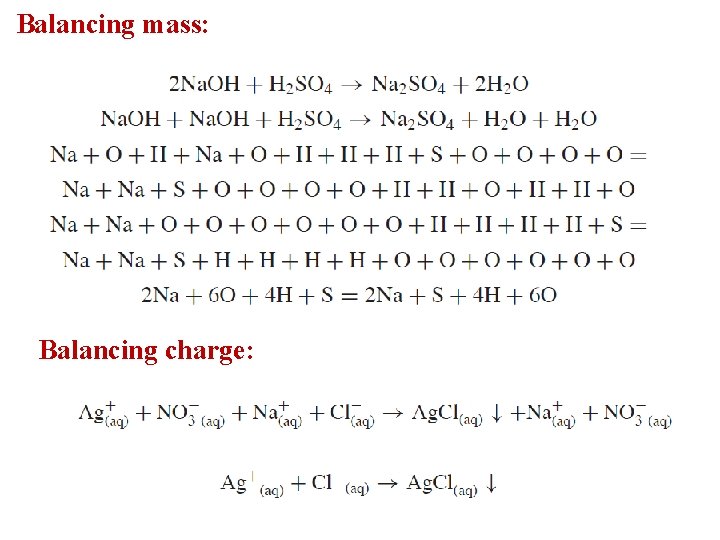
Balancing mass: Balancing charge:

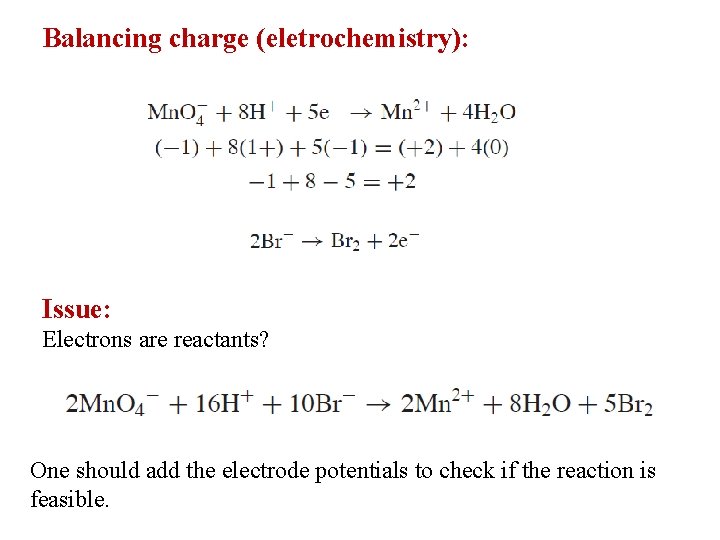
Balancing charge (eletrochemistry): Issue: Electrons are reactants? One should add the electrode potentials to check if the reaction is feasible.

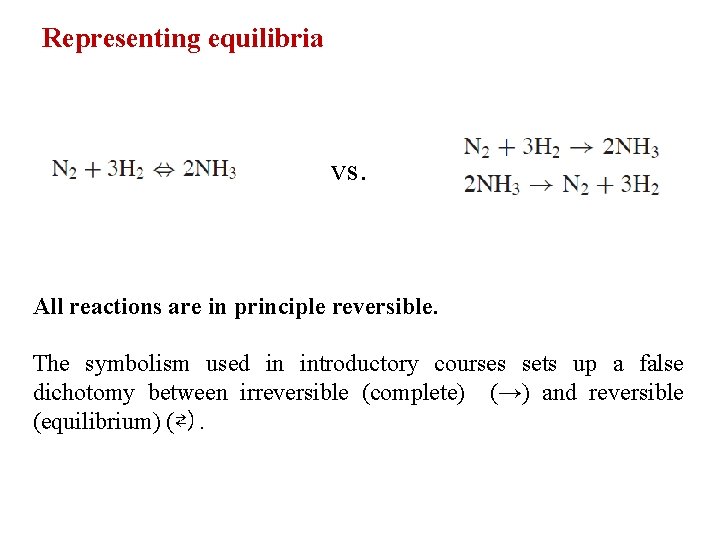
Representing equilibria vs. All reactions are in principle reversible. The symbolism used in introductory courses sets up a false dichotomy between irreversible (complete) (→) and reversible (equilibrium) (⇄).

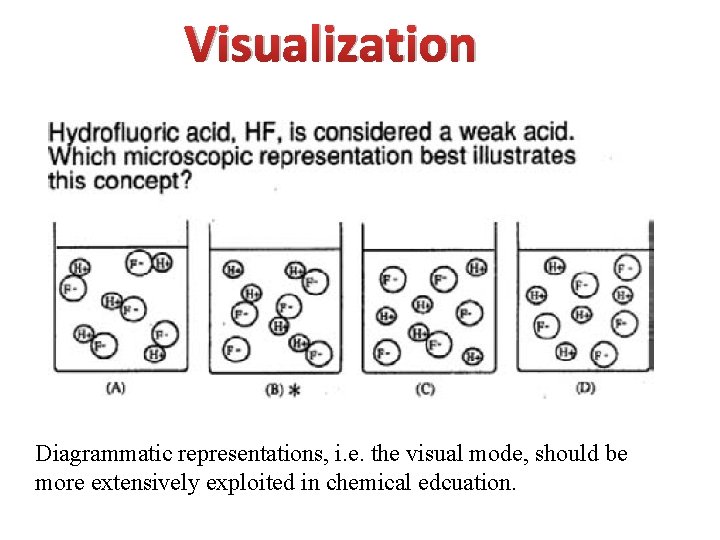
Visualization Diagrammatic representations, i. e. the visual mode, should be more extensively exploited in chemical edcuation.

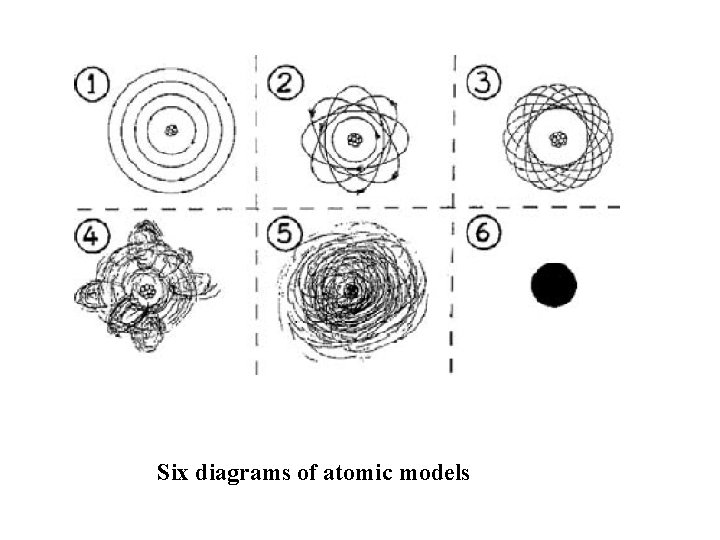
Six diagrams of atomic models

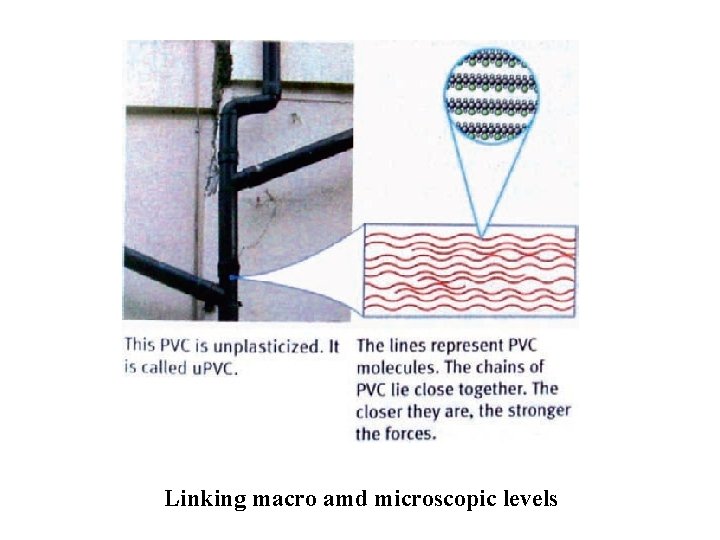
Linking macro amd microscopic levels

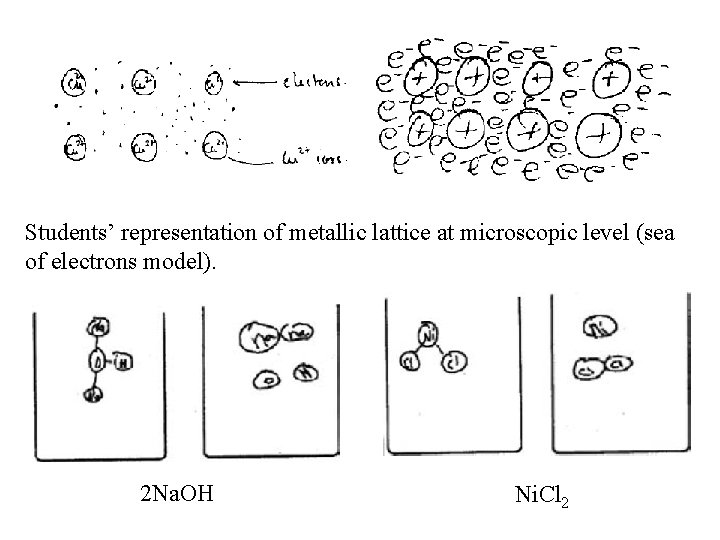
Students’ representation of metallic lattice at microscopic level (sea of electrons model). 2 Na. OH Ni. Cl 2

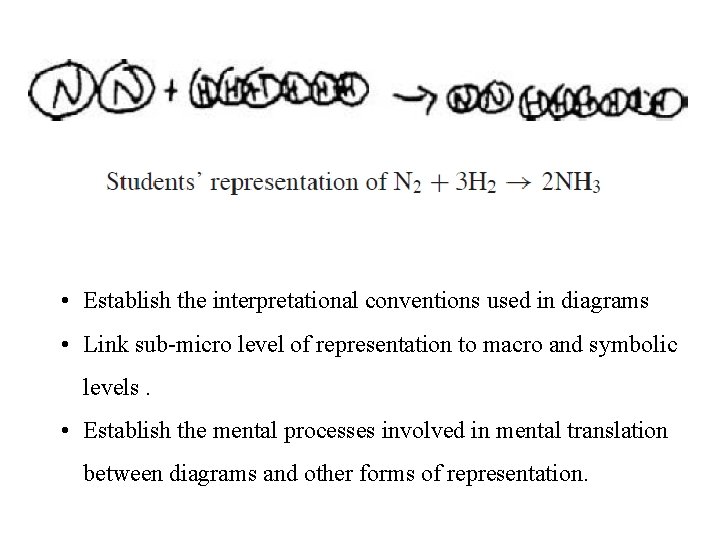
• Establish the interpretational conventions used in diagrams • Link sub-micro level of representation to macro and symbolic levels. • Establish the mental processes involved in mental translation between diagrams and other forms of representation.

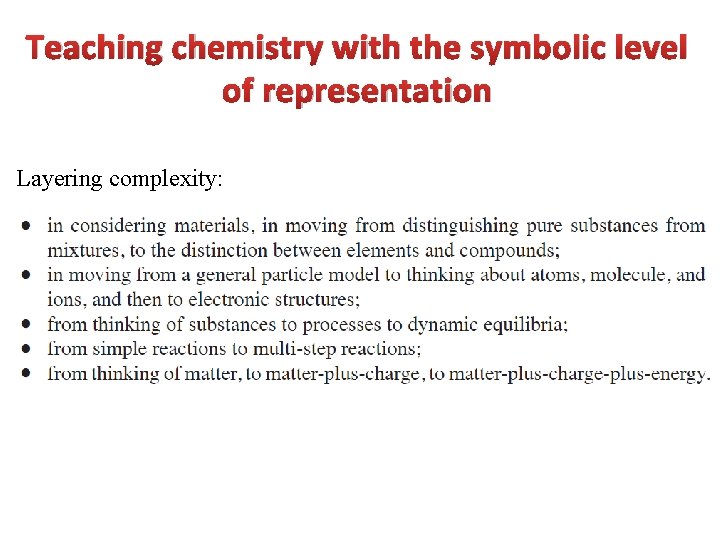
Teaching chemistry with the symbolic level of representation Layering complexity:

The teachers should: Teachers should bear in mind the need to support progression between increasing levels of complexity.



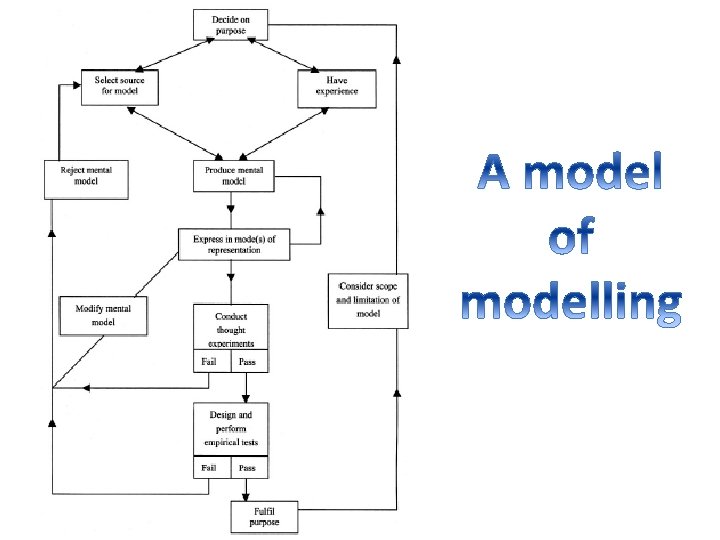
Learning models • Coming to know the major models produced by chemists • Coming to know the scope and limitations of such models • Appreciating the role of models in the accreditation and dissemination of the products of chemical enquiry • Creating and testing models

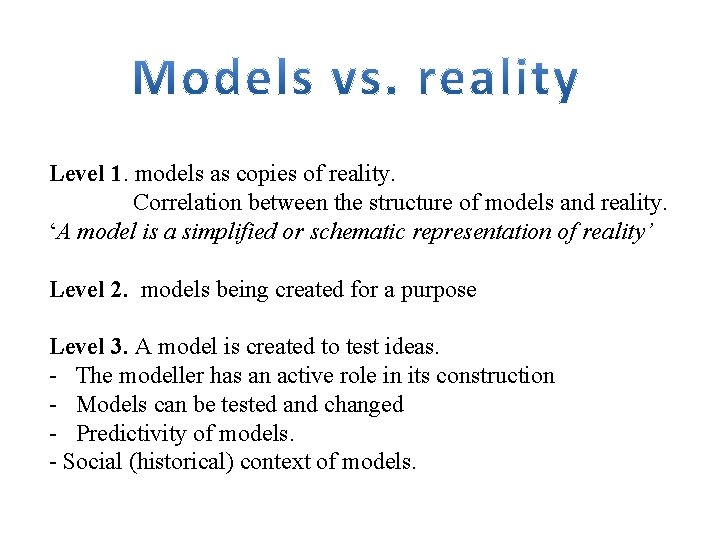
Level 1. models as copies of reality. Correlation between the structure of models and reality. ‘A model is a simplified or schematic representation of reality’ Level 2. models being created for a purpose Level 3. A model is created to test ideas. - The modeller has an active role in its construction - Models can be tested and changed - Predictivity of models. - Social (historical) context of models.

The use of a diversity of models (at different levels of explanations) for a given reality is generally challenging and confusing for students. Role of computational model for visualization and representation (ex. Chemical bond, molecular structure…. . )

The teacher’s perspective • How the students construct their own mental models? • How these models can be constructively used in class? • How to generate scientific consensus model?

Although overabundance of models are generally found in the chemistry textbooks, they usually do not even discuss the meaning of ‘models’. They tend to present science as a collection of true or complete facts.


Good practice • Explicitly introduce the representational conventions in use • Avoid the use of hybrid models • Provide opportunities for students to develop and test their own models • Introduce the students to the ‘nature of model’ • State scope and limitations of the model in use



