modelling drying and particle formation in spray towers



- Slides: 27

modelling drying and particle formation in spray towers Christopher Handscomb Wednesday 9 th May 2007

outline • Introduction to spray drying • Modelling approach • Continuous phase gas flow • Single particle drying • Conclusions and further work Christopher Handscomb (csh 33@cam. ac. uk)

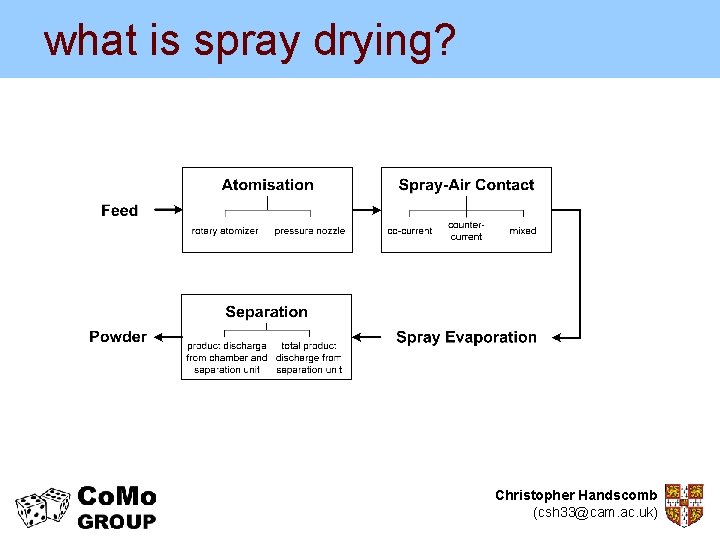
what is spray drying? • An important technology in industry • Used to produce, for example: – Pharmaceuticals – Food stuffs (e. g. milk powder and coffee) – Detergents • Unique drying technology combining moisture removal and particle formation Christopher Handscomb (csh 33@cam. ac. uk)

what is spray drying? Christopher Handscomb (csh 33@cam. ac. uk)

motivation A computational model would… • predict the effect of process conditions on final product properties • guide the operator towards safe and efficient operating conditions • facilitate the design of new plant based on physics, rather than correlations Christopher Handscomb (csh 33@cam. ac. uk)

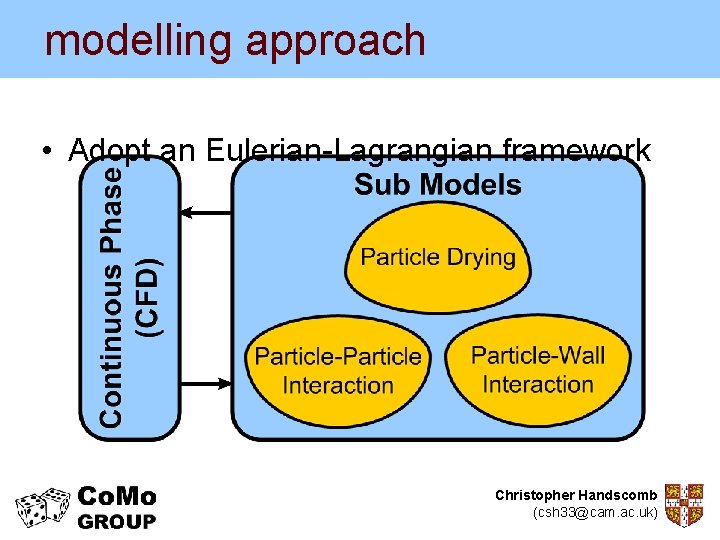
modelling approach • Adopt an Eulerian-Lagrangian framework Christopher Handscomb (csh 33@cam. ac. uk)

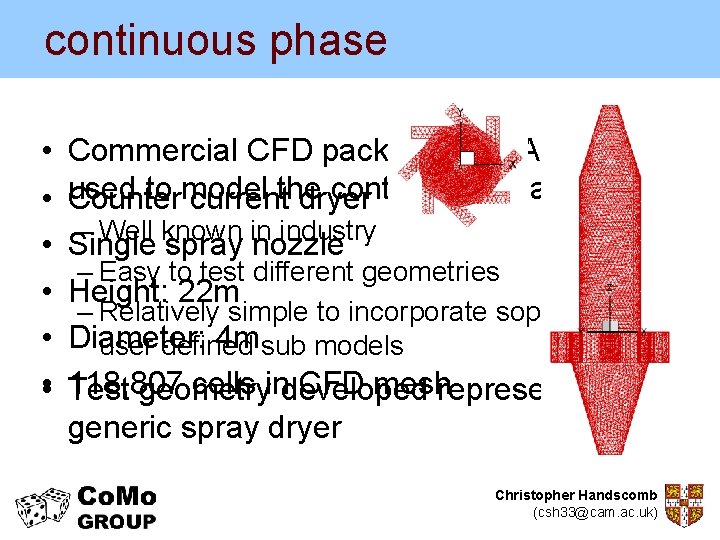
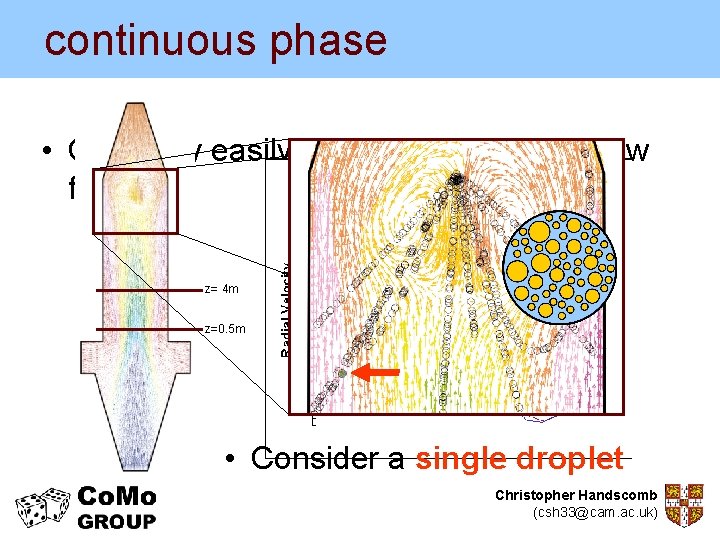
continuous phase • • • Commercial CFD package – STAR CD – used to model continuous phase Counter currentthe dryer – Well known in industry Single spray nozzle – Easy to test different geometries Height: 22 m – Relatively simple to incorporate sophisticated Diameter: 4 m sub models user defined 118, 807 cells indeveloped CFD meshrepresenting a Test geometry generic spray dryer Christopher Handscomb (csh 33@cam. ac. uk)

continuous phase • Can fairly easily produce plots of the flow field z= 4 m z=0. 5 m • Consider a single droplet Christopher Handscomb (csh 33@cam. ac. uk)

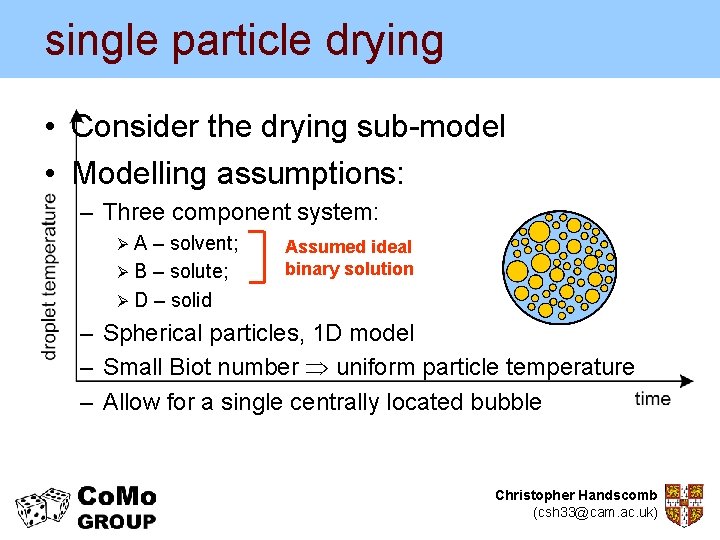
single particle drying • Consider the drying sub-model • Modelling assumptions: – Three component system: ØA – solvent; Ø B – solute; Ø D – solid Assumed ideal binary solution – Spherical particles, 1 D model – Small Biot number uniform particle temperature – Allow for a single centrally located bubble Christopher Handscomb (csh 33@cam. ac. uk)

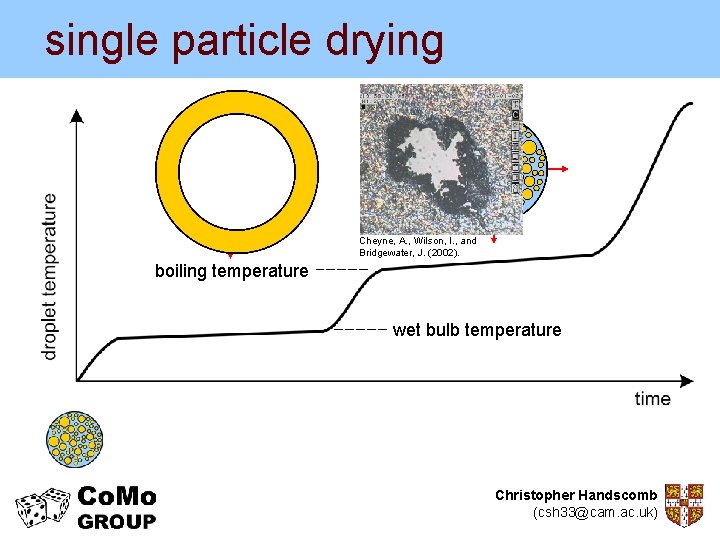
single particle drying Cheyne, A. , Wilson, I. , and Bridgewater, J. (2002). boiling temperature wet bulb temperature Christopher Handscomb (csh 33@cam. ac. uk)

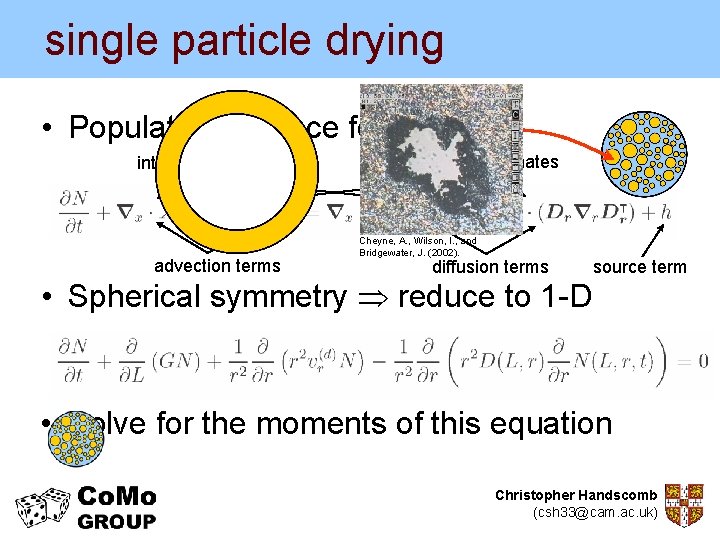
single particle drying • Population balance for solids internal coordinates advection terms external coordinates Cheyne, A. , Wilson, I. , and Bridgewater, J. (2002). diffusion terms • Spherical symmetry reduce to 1 -D source term • Solve for the moments of this equation Christopher Handscomb (csh 33@cam. ac. uk)

single particle drying • Variable of interest is solids volume fraction • Related to the moments of the population balance equation by: • Obtained by solving the moment system: assumed independent of internal coordinate (particle size) Christopher Handscomb (csh 33@cam. ac. uk)

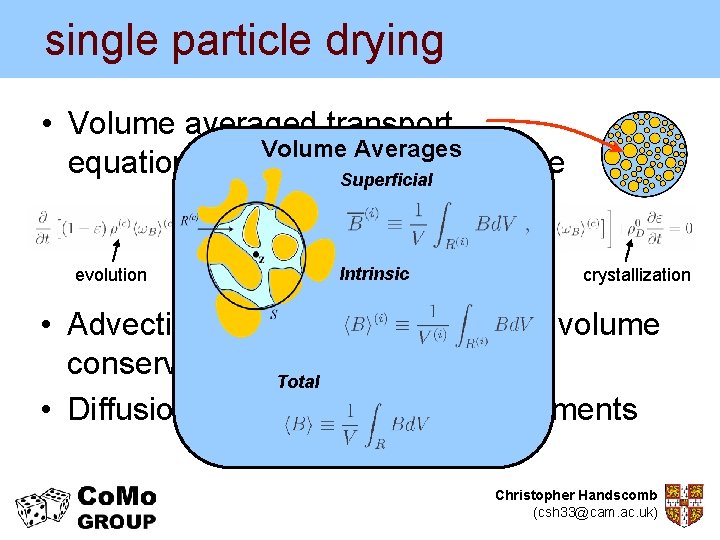
single particle drying • Volume averaged transport Volume Averages equations for the continuous phase Superficial evolution advection Intrinsic diffusion crystallization • Advection velocity calculated from volume conservation considerations Total • Diffusion coefficient from measurements Christopher Handscomb (csh 33@cam. ac. uk)

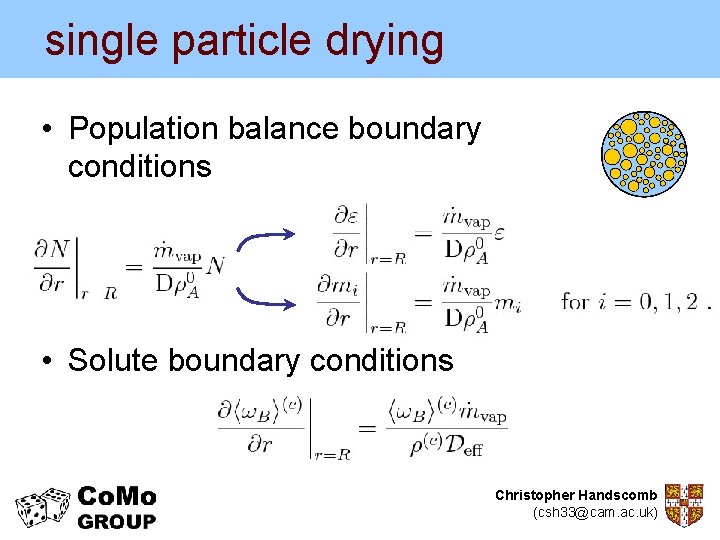
single particle drying • Population balance boundary conditions • Solute boundary conditions Christopher Handscomb (csh 33@cam. ac. uk)

moving boundary • Moving boundary handled through a standard coordinate transformation r z: • This adds a ‘virtual flux’ to all equations virtual flux Christopher Handscomb (csh 33@cam. ac. uk)

solution method • Problem is a system of PDEs and coupled ODEs • Solved using Numerical Algorithms Group (NAG) library routines for convection-diffusion type equations • Finite Volume approach with user-defined flux function Christopher Handscomb (csh 33@cam. ac. uk)

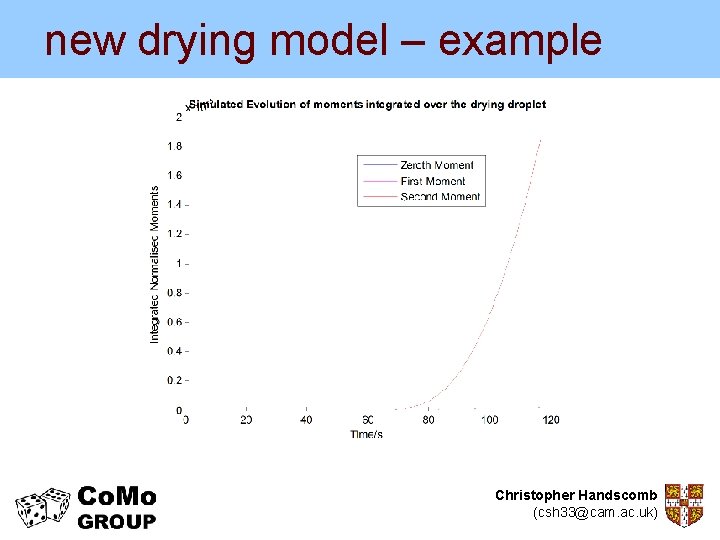
new drying model – example • Model described so far can simulate up to the point of shell formation • e. g. Consider a system: – Initial 14 wt% sodium sulphate solution – no solids – Crystallisation model from Rosenblatt et al. (1984): ‘Kinetics of Phase Transitions in the System Sodium Sulphate-Water’ – Droplet diameter = 1. 78 mm – Drying air T = 373 K – Droplets initially well mixed Christopher Handscomb (csh 33@cam. ac. uk)

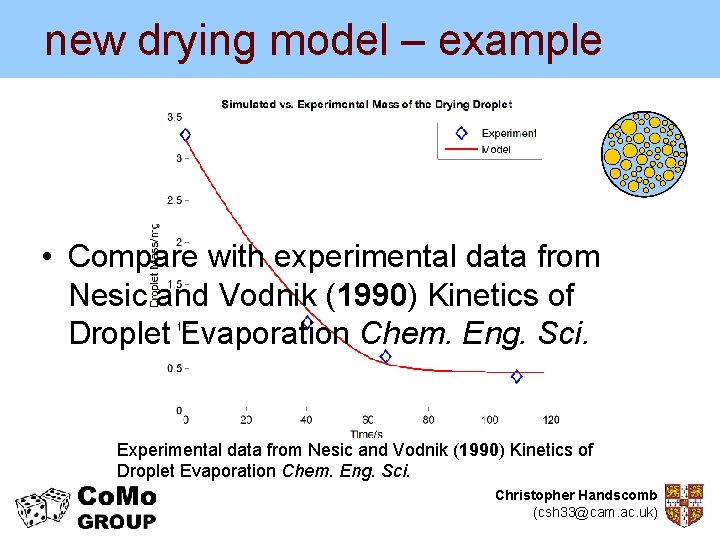
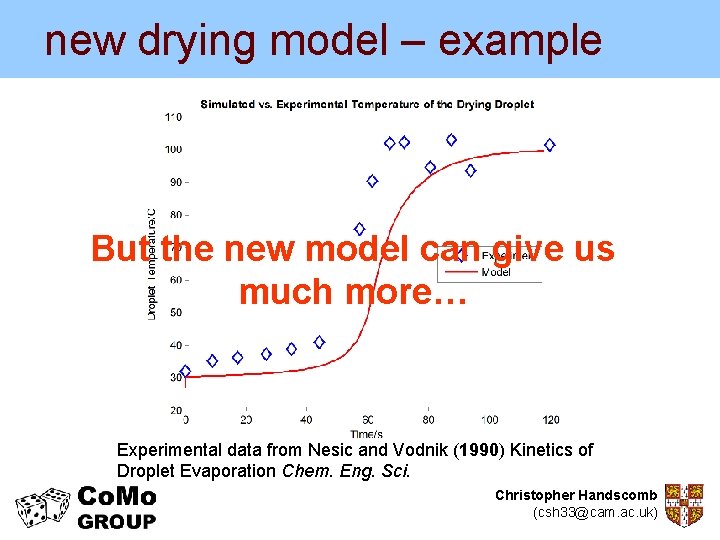
new drying model – example • Compare with experimental data from Nesic and Vodnik (1990) Kinetics of Droplet Evaporation Chem. Eng. Sci. Experimental data from Nesic and Vodnik (1990) Kinetics of Droplet Evaporation Chem. Eng. Sci. Christopher Handscomb (csh 33@cam. ac. uk)

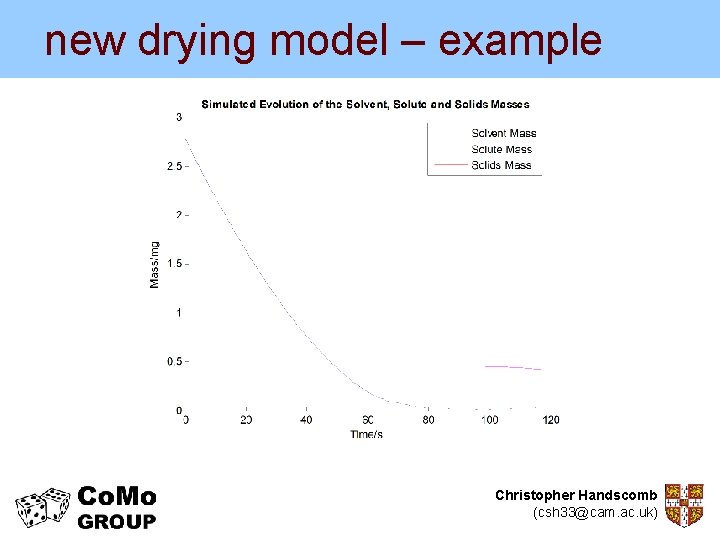
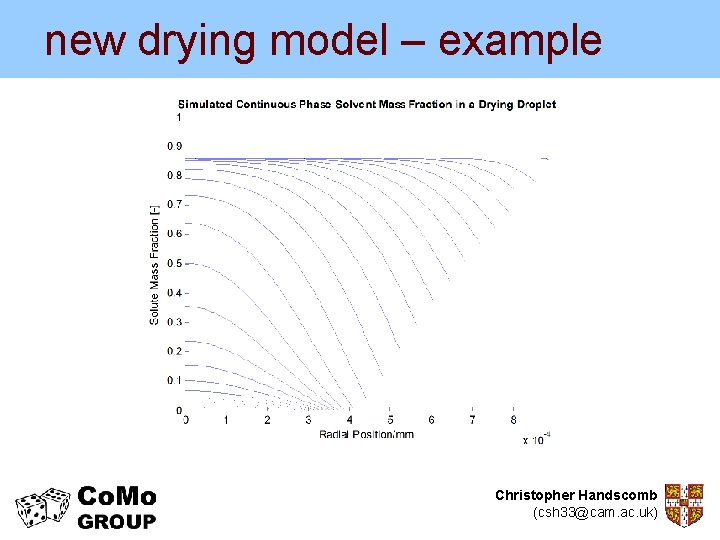
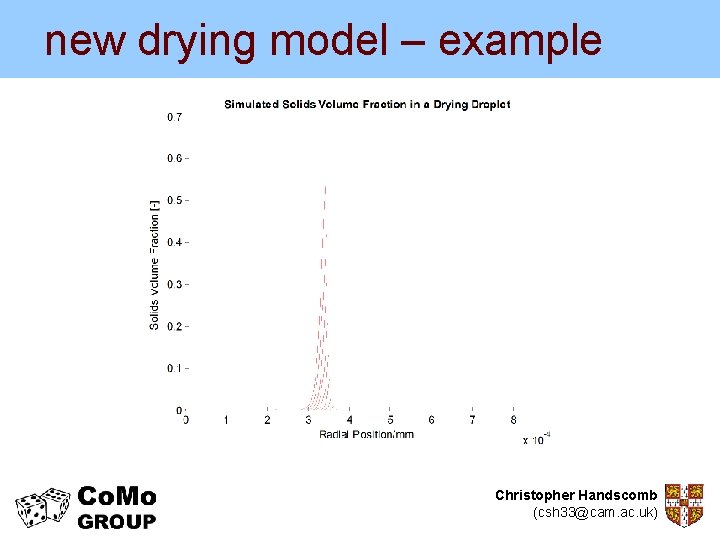
new drying model – example But the new model can give us much more… Experimental data from Nesic and Vodnik (1990) Kinetics of Droplet Evaporation Chem. Eng. Sci. Christopher Handscomb (csh 33@cam. ac. uk)

new drying model – example Christopher Handscomb (csh 33@cam. ac. uk)

new drying model – example Christopher Handscomb (csh 33@cam. ac. uk)

new drying model – example Christopher Handscomb (csh 33@cam. ac. uk)

new drying model – example Christopher Handscomb (csh 33@cam. ac. uk)

conclusions… • Introduction to spray drying and the associated modelling challenges • Results of continuous phase simulation • Overview of a new drying model • Comparison with experiments for a ‘simple’ case… Christopher Handscomb (csh 33@cam. ac. uk)

…work not shown… • Drying after shell formation • Simulation of detergent droplets drying with experimental comparison • Simplified drying models implemented in CFD code Christopher Handscomb (csh 33@cam. ac. uk)

…and further work • Obtain data and validate model for high temperature drying • Couple (simplified) model to CFD simulation • Compare with existing drying models when used in CFD Christopher Handscomb (csh 33@cam. ac. uk)

acknowledgements Christopher Handscomb (csh 33@cam. ac. uk)