Modeling Data Methods and Examples Arthur G Roberts

Modeling Data: Methods and Examples Arthur G. Roberts

WHAT IS MODELING?
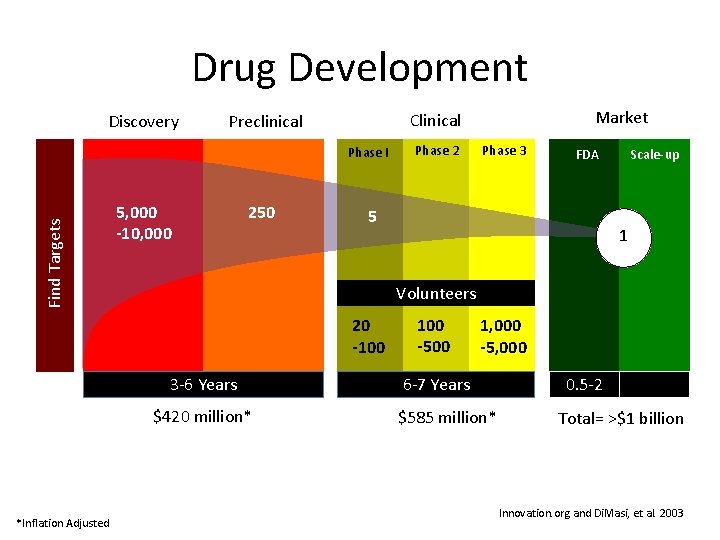
Drug Development Discovery Find Targets Phase I 5, 000 -10, 000 250 Phase 2 Phase 3 FDA 5 Scale-up 1 Volunteers 20 -100 3 -6 Years $420 million* *Inflation Adjusted Market Clinical Preclinical 100 -500 1, 000 -5, 000 6 -7 Years $585 million* 0. 5 -2 Total= >$1 billion Innovation. org and Di. Masi, et al. 2003
Drug Development
Outline • Model Types – PK/PD – Disease Progression – Meta-models and Bayesian Averaging – Population • Estimating Parameters • Simulation Methods • Regulatory Aspects
![PK models • [drug] versus time • types – compartment PK modeling (CPK) – PK models • [drug] versus time • types – compartment PK modeling (CPK) –](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-6.jpg)
PK models • [drug] versus time • types – compartment PK modeling (CPK) – physiology-based PK modeling (PBPK)

PK models: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible
![PK models: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible [Drug] PK models: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible [Drug]](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-8.jpg)
PK models: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible [Drug]
![CPK: Topology • • • Closed [Drug] Open Catenary Cyclic Mammillary Reducible in [Drug]out CPK: Topology • • • Closed [Drug] Open Catenary Cyclic Mammillary Reducible in [Drug]out](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-9.jpg)
CPK: Topology • • • Closed [Drug] Open Catenary Cyclic Mammillary Reducible in [Drug]out
![CPK: Topology • • • Closed Open Catenary [Drug] Cyclic Mammillary Reducible Compartment 1 CPK: Topology • • • Closed Open Catenary [Drug] Cyclic Mammillary Reducible Compartment 1](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-10.jpg)
CPK: Topology • • • Closed Open Catenary [Drug] Cyclic Mammillary Reducible Compartment 1 in [Drug] Compartment 2 [Drug] Chain Compartment 3 [Drug]out
![CPK: Topology • • • Closed Open Catenary [Drug] Cyclic Mammillary Reducible Compartment 1 CPK: Topology • • • Closed Open Catenary [Drug] Cyclic Mammillary Reducible Compartment 1](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-11.jpg)
CPK: Topology • • • Closed Open Catenary [Drug] Cyclic Mammillary Reducible Compartment 1 in [Drug] Compartment 2 [Drug] Compartment 3 [Drug]out
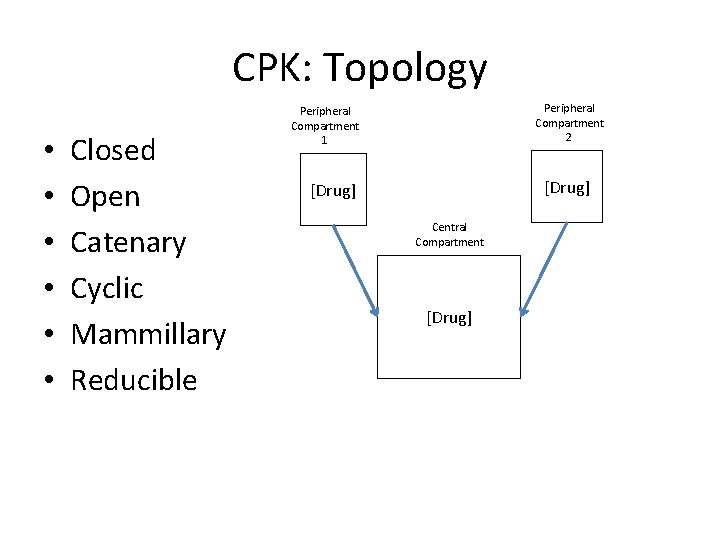
CPK: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible Peripheral Compartment 2 Peripheral Compartment 1 [Drug] Central Compartment [Drug]
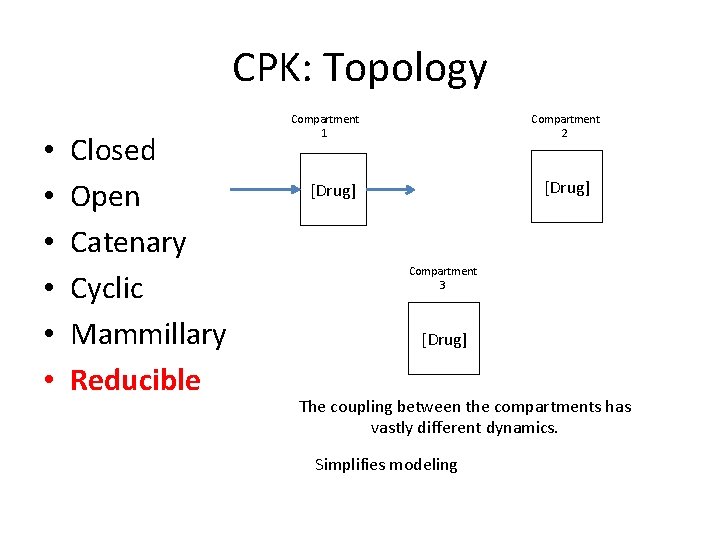
CPK: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible Compartment 2 Compartment 1 [Drug] Compartment 3 [Drug] The coupling between the compartments has vastly different dynamics. Simplifies modeling
![CPK: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible Brain [Drug]Receptor [Drug] CPK: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible Brain [Drug]Receptor [Drug]](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-14.jpg)
CPK: Topology • • • Closed Open Catenary Cyclic Mammillary Reducible Brain [Drug]Receptor [Drug] Elimination Response Liver
![CPK: Topology • • • Closed [Drug] Open Catenary [Drug] Cyclic Mammillary Liver Reducible CPK: Topology • • • Closed [Drug] Open Catenary [Drug] Cyclic Mammillary Liver Reducible](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-15.jpg)
CPK: Topology • • • Closed [Drug] Open Catenary [Drug] Cyclic Mammillary Liver Reducible Brain [Drug]Receptor Elimination Response

Physiology-Based PK
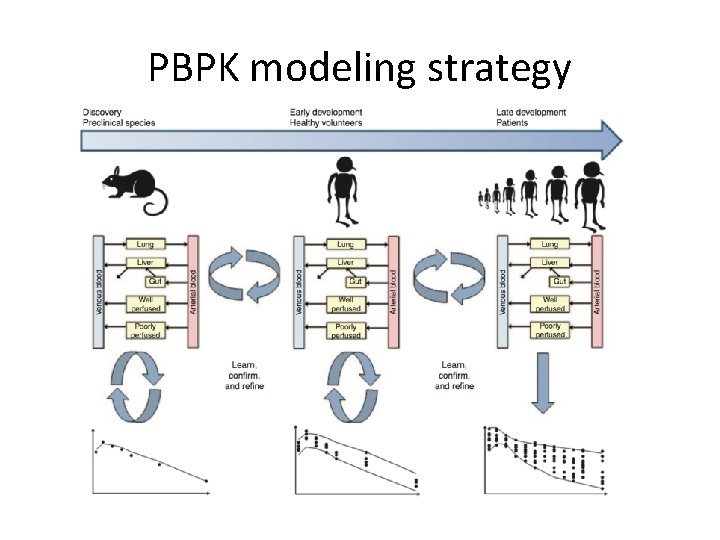
PBPK modeling strategy
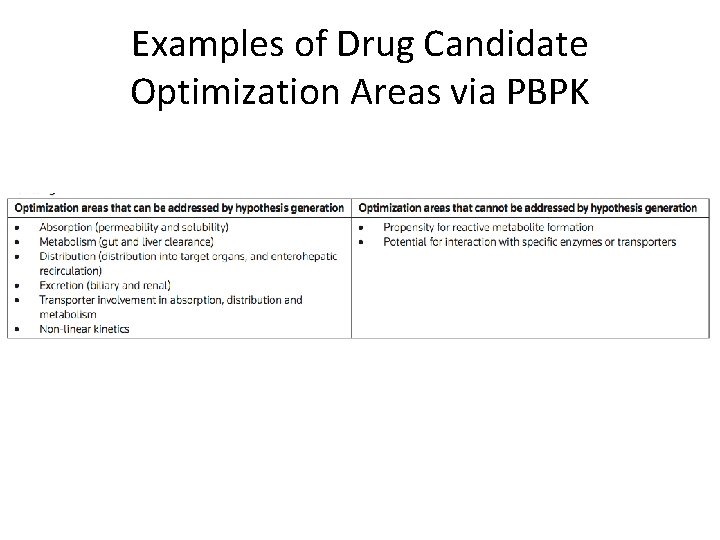
Examples of Drug Candidate Optimization Areas via PBPK
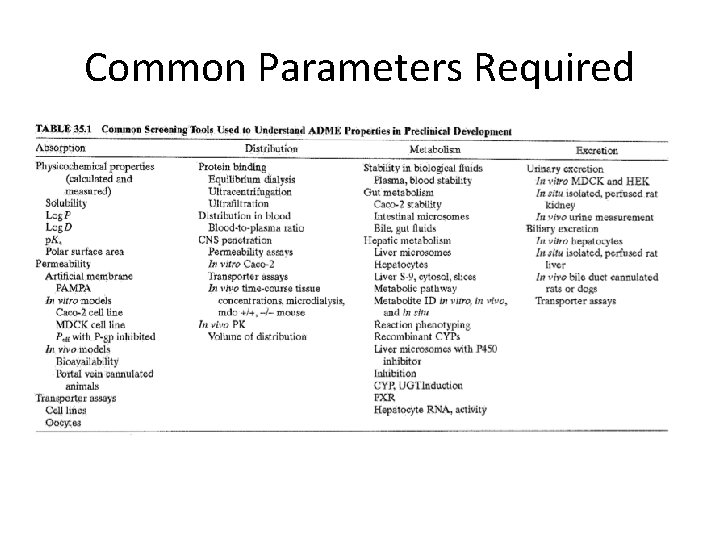
Common Parameters Required
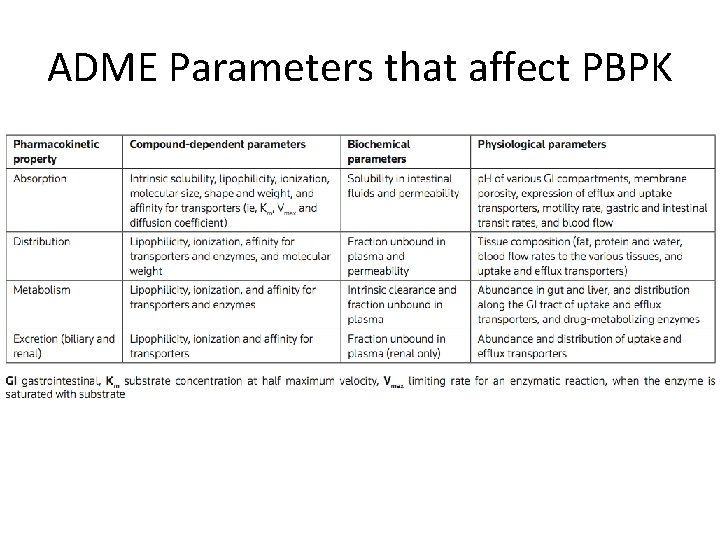
ADME Parameters that affect PBPK

Where PBPK add value or fail
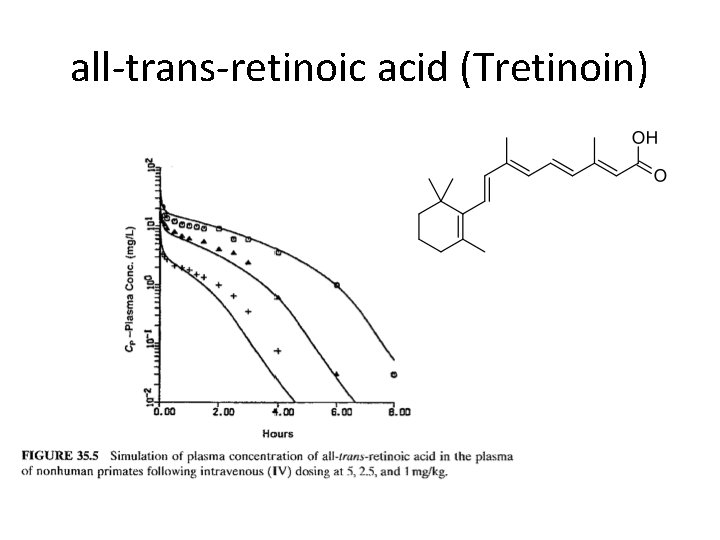
all-trans-retinoic acid (Tretinoin)
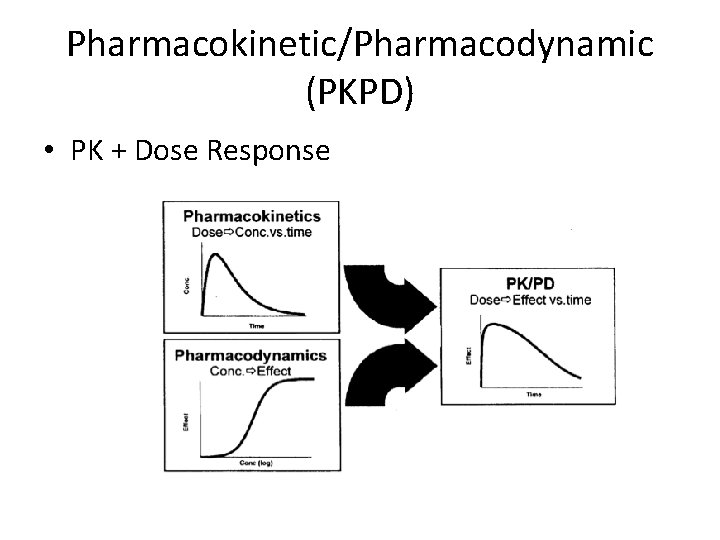
Pharmacokinetic/Pharmacodynamic (PKPD) • PK + Dose Response
Pharmacokinetic/Pharmacodynamic Modelling • Procedure – Estimate exposure – Correlate exposure to PD or other endpoints (e. g. excretion rates) – Use mechanistic models – Model excretion rate as a function of exposure • Purpose – Estimate therapeutic window – Dose selection – Mechanism
PD Models • Steady-state • Non-steady state
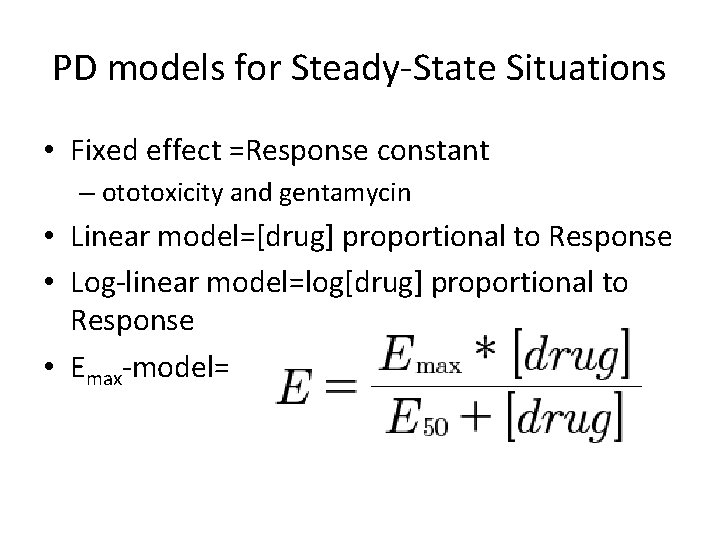
PD models for Steady-State Situations • Fixed effect =Response constant – ototoxicity and gentamycin • Linear model=[drug] proportional to Response • Log-linear model=log[drug] proportional to Response • Emax-model=
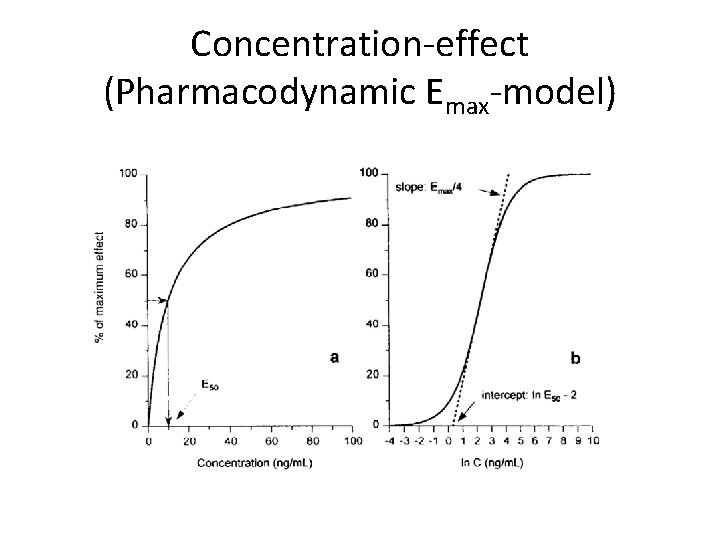
Concentration-effect (Pharmacodynamic Emax-model)
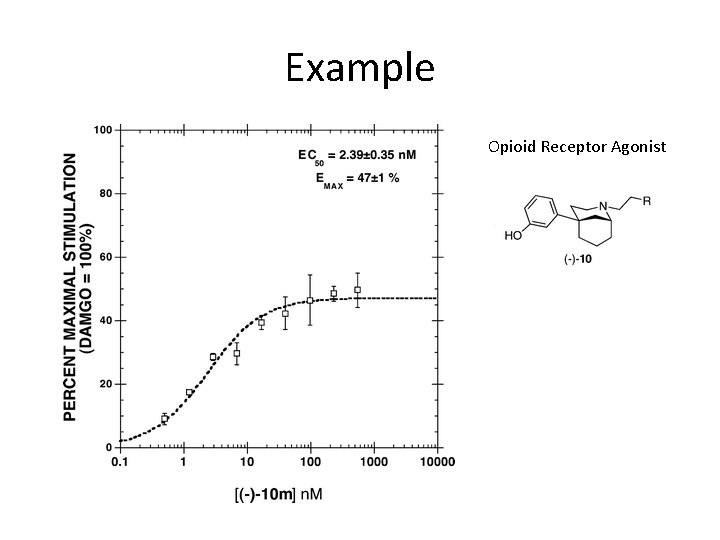
Example Opioid Receptor Agonist
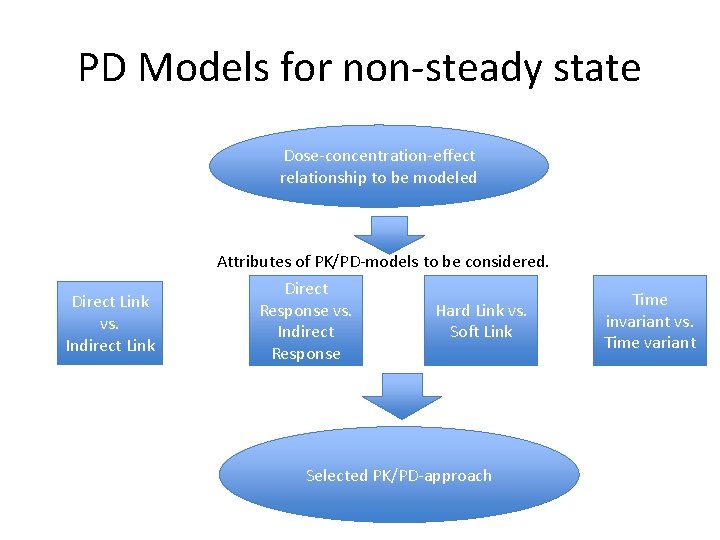
PD Models for non-steady state Dose-concentration-effect relationship to be modeled Attributes of PK/PD-models to be considered. Direct Link vs. Indirect Link Direct Response vs. Indirect Response Hard Link vs. Soft Link Selected PK/PD-approach Time invariant vs. Time variant
![Direct link versus indirect link Direct Link Brain Plasma Elimination [Drug] Relative concentrations between Direct link versus indirect link Direct Link Brain Plasma Elimination [Drug] Relative concentrations between](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-30.jpg)
Direct link versus indirect link Direct Link Brain Plasma Elimination [Drug] Relative concentrations between the plasma and the brain remain relatively constant despite the system not being in steady-state. Indirect Link Exhibit hysteresis Distribution delay
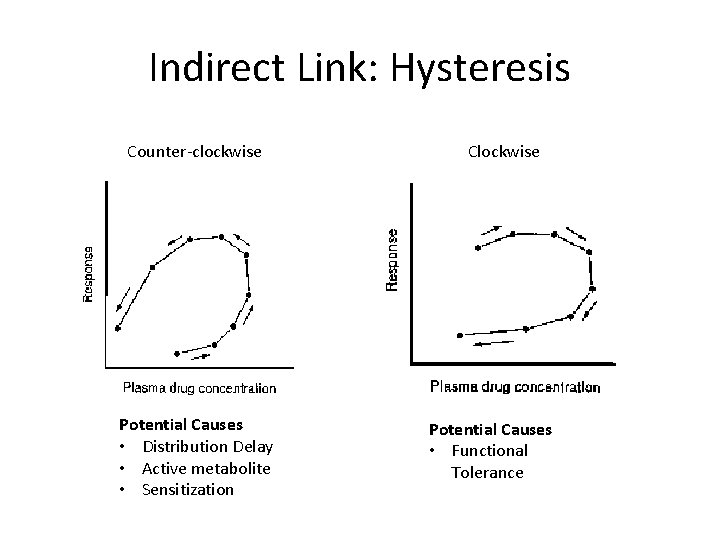
Indirect Link: Hysteresis Counter-clockwise Potential Causes • Distribution Delay • Active metabolite • Sensitization Clockwise Potential Causes • Functional Tolerance
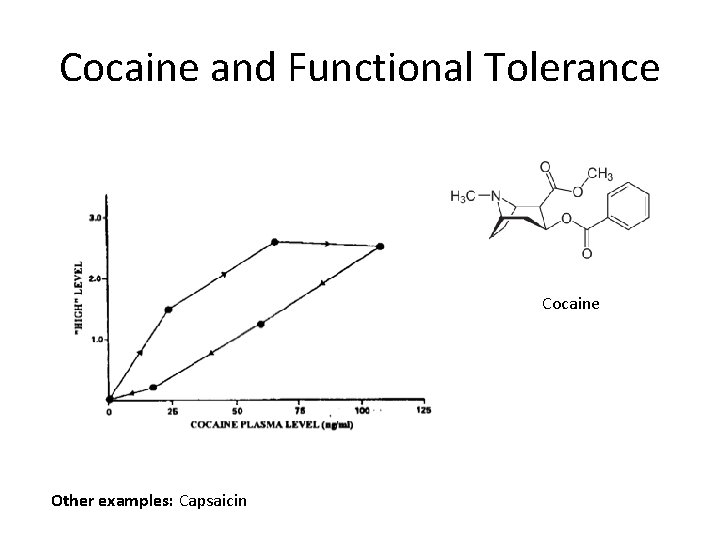
Cocaine and Functional Tolerance Cocaine Other examples: Capsaicin
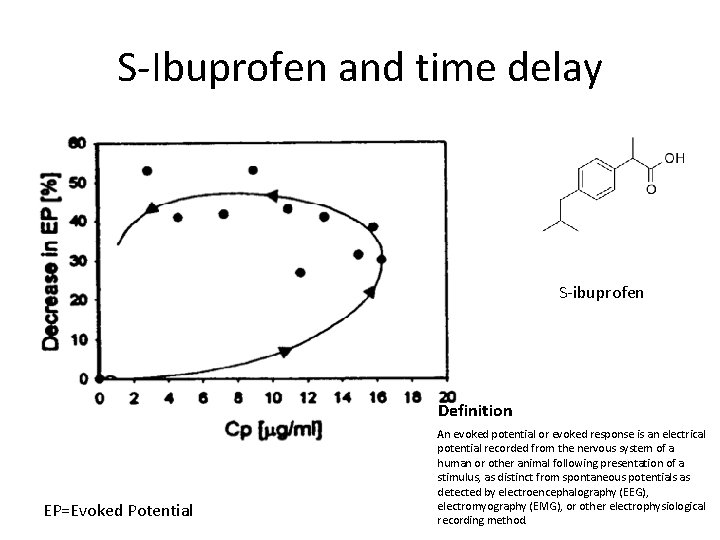
S-Ibuprofen and time delay S-ibuprofen Definition EP=Evoked Potential An evoked potential or evoked response is an electrical potential recorded from the nervous system of a human or other animal following presentation of a stimulus, as distinct from spontaneous potentials as detected by electroencephalography (EEG), electromyography (EMG), or other electrophysiological recording method.
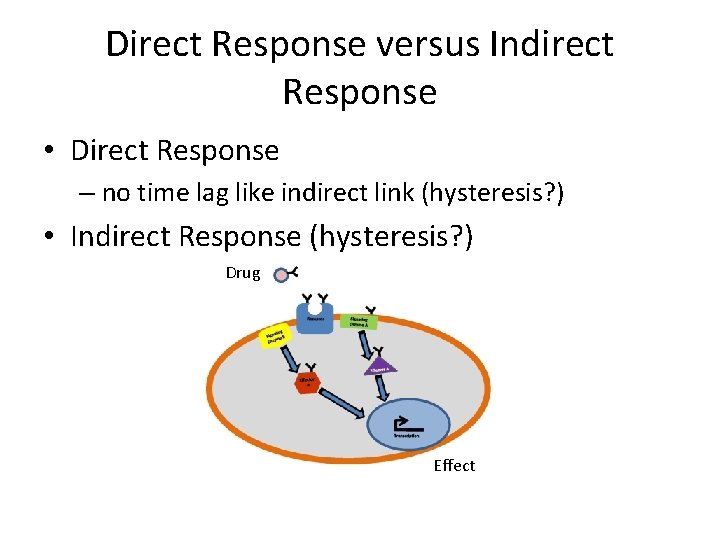
Direct Response versus Indirect Response • Direct Response – no time lag like indirect link (hysteresis? ) • Indirect Response (hysteresis? ) Drug Effect
![Indirect Response Lymphocytes [drug]P fluticasone Indirect Response Lymphocytes [drug]P fluticasone](http://slidetodoc.com/presentation_image_h/0c4a1622a2d130ceda9f8acab967771f/image-35.jpg)
Indirect Response Lymphocytes [drug]P fluticasone
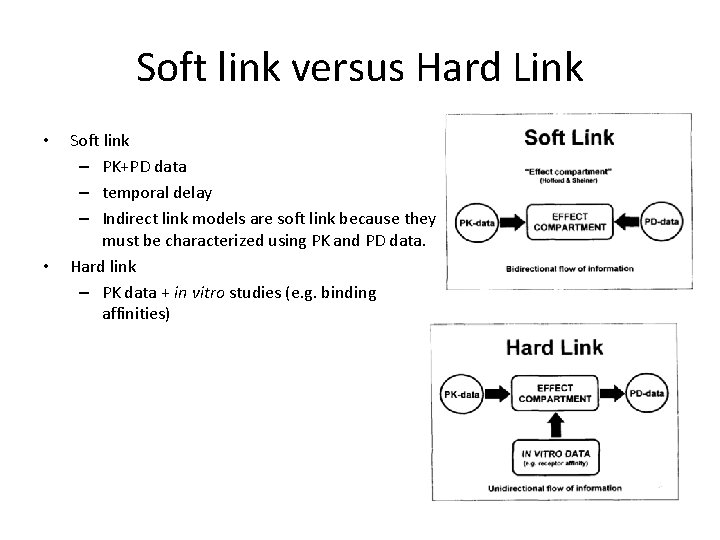
Soft link versus Hard Link • • Soft link – PK+PD data – temporal delay – Indirect link models are soft link because they must be characterized using PK and PD data. Hard link – PK data + in vitro studies (e. g. binding affinities)

Time variant versus time invariant • Tolerance – Functional or PD tolerance (Hysteresis? ) • Sensitization (Hysteresis)
Disease Progression Models • 1992 – Alzheimer’s via Alzheimer Disease Assessment Scale (ADASC) • Characteristics – Subject variability – Correlated to PK model – Drug effects
Meta-models and Bayesian averaging • Meta-analyses means “the analysis of analyses” • Bayesian averaging – Thomas Bayes (1702 -1761) – Biased averaged based on other information – Method to average several different models
Population Models • Data and database preparation • Structural models – algebraic equations – differential equations • Linearity and superposition • Stochastic models for random effects • Covariate models for fixed effects
Population Models: Data and database preparation • • only good as the data in them accuracy (remove errors) data consistency remove significant outliers
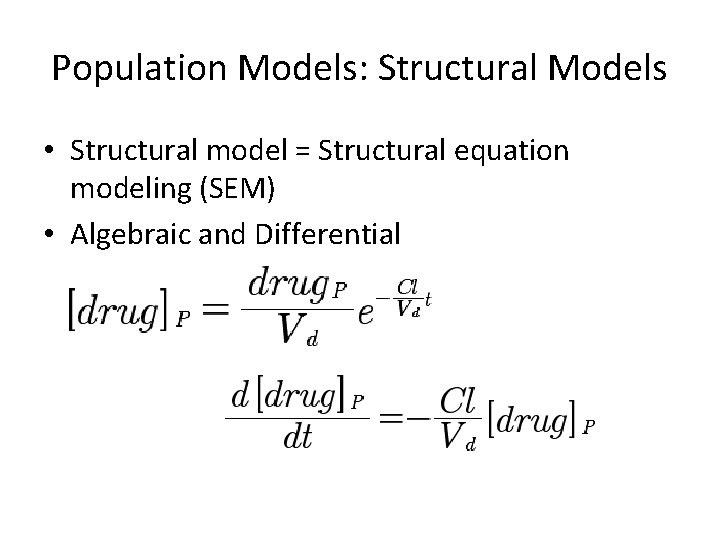
Population Models: Structural Models • Structural model = Structural equation modeling (SEM) • Algebraic and Differential
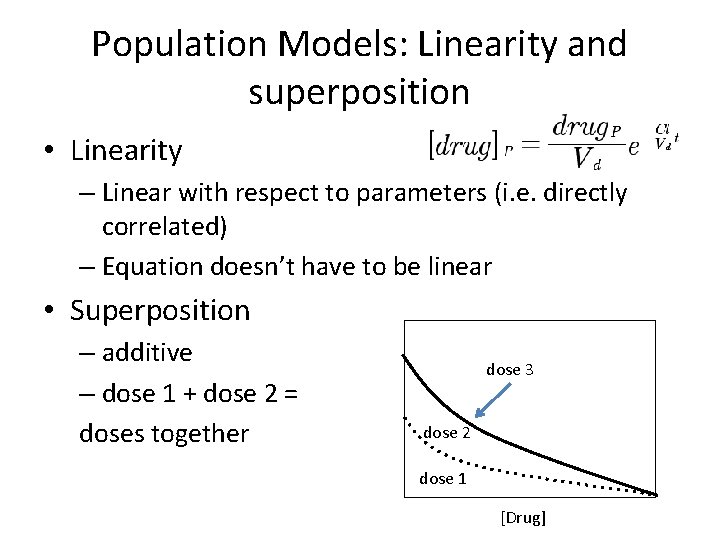
Population Models: Linearity and superposition • Linearity – Linear with respect to parameters (i. e. directly correlated) – Equation doesn’t have to be linear • Superposition – additive – dose 1 + dose 2 = doses together dose 3 dose 2 dose 1 [Drug]
Population Models: Stochastic Models for Random Effects • Variability – low therapeutic index high probability of subtherapeutic and toxic exposure – Residual unexplained variability (RUV) • Observation value – Model predicted value – Between subject variability (BSV) – 1 level-linear regression – Multi-level-hierarchies
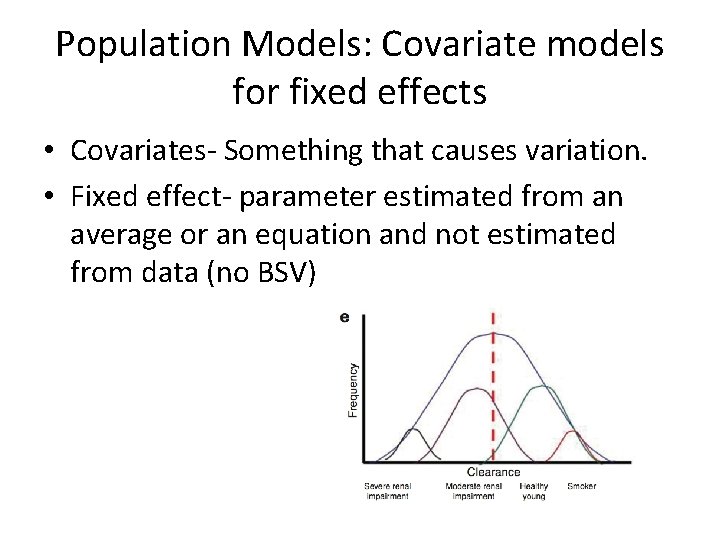
Population Models: Covariate models for fixed effects • Covariates- Something that causes variation. • Fixed effect- parameter estimated from an average or an equation and not estimated from data (no BSV)
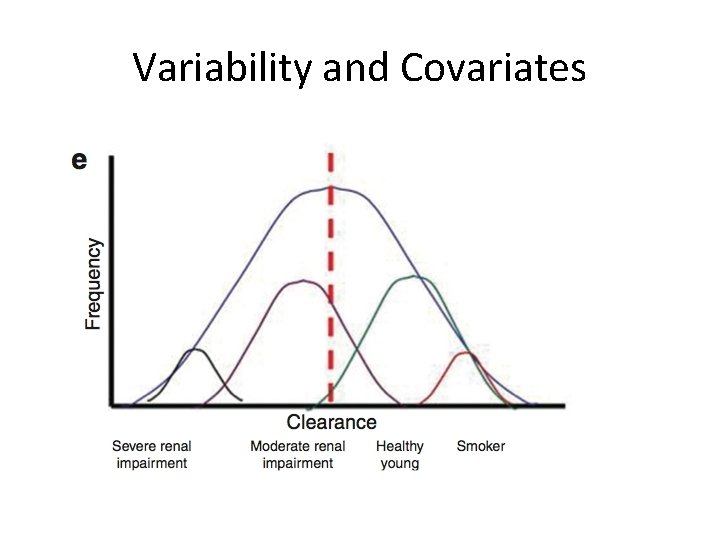
Variability and Covariates
Estimating Parameters • Least Squares – slope and intercept values – residues=Value-Average Value – least squares= Sum of (Value-Average Value)^2 • Weights – least squares weighted toward high data points • Objective Function Value (OFV) – sum of squared deviations between the predictions and the observations or -2*log(likelihood) – likelihood = sum of squares for simple problems. – minimum value = best fit • Parameter Optimization – used because PK has too many variables
Parameter Optimization Examples • • Evolutionary Programming Genetic Algorithm Simulated Annealing Random Searching
Simulation Methods • Validation – internal – subset of the data – external – new data set • Extrapolation – simulating data outside the observed data set • Limitations and Assumptions • Non-Stochastic Simulations (simple fitting) • Stochastic Simulations – Random-effect parameters (e. g. Population Variability) • simulated with a random number generator based on a distribution – Model simulated repeatedly
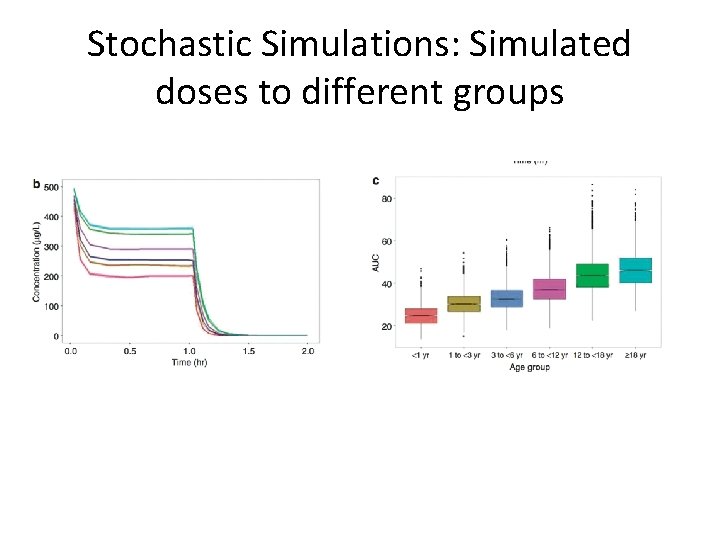
Stochastic Simulations: Simulated doses to different groups

Simulation Software • Proprietary – PK-Sim 5 – Pheonix Win. Nonlin • Freeish – Monolix • http: //www. lixoft. eu/products/monolix/product-monolixoverview/ – Excel • Open Source or Free – http: //www. pharmpk. com/soft. html • Java. PK for Desktop
Regulatory Aspects • FDA Modernization Act of 1997 – exposure-response with a single clinical trial = effectiveness – Population modeling • identify sources of variability safety and efficacy • Personalized Medicine – Cost effective – Modeling critical • Optimize doses – Pharmacogenetics • Warfarin exposure and response dependent on CYP 2 C 9 genotrype

END OF MODELING DATA AND EXAMPLES
- Slides: 53