Mobius HD A selfexpanding carotid implant William A

Mobius. HD: A self-expanding carotid implant William A. Gray MD System Chief of Cardiovascular Services, Main Line Health President, Lankenau Heart Institute Wynnewood, PA USA
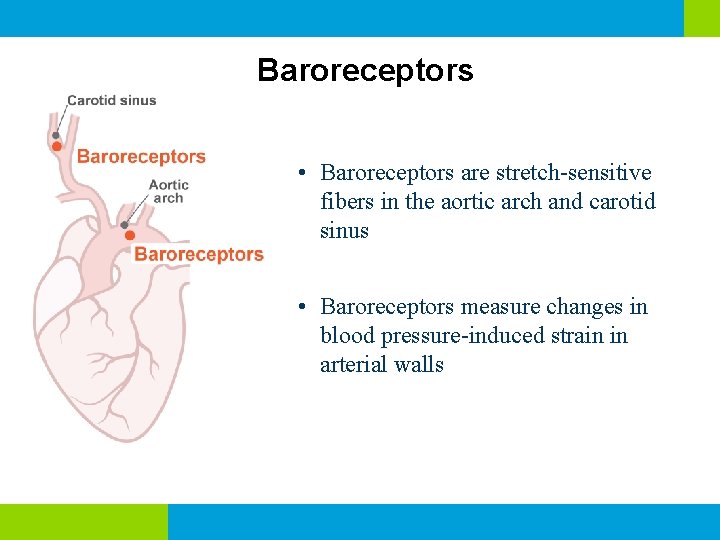
Baroreceptors • Baroreceptors are stretch-sensitive fibers in the aortic arch and carotid sinus • Baroreceptors measure changes in blood pressure-induced strain in arterial walls
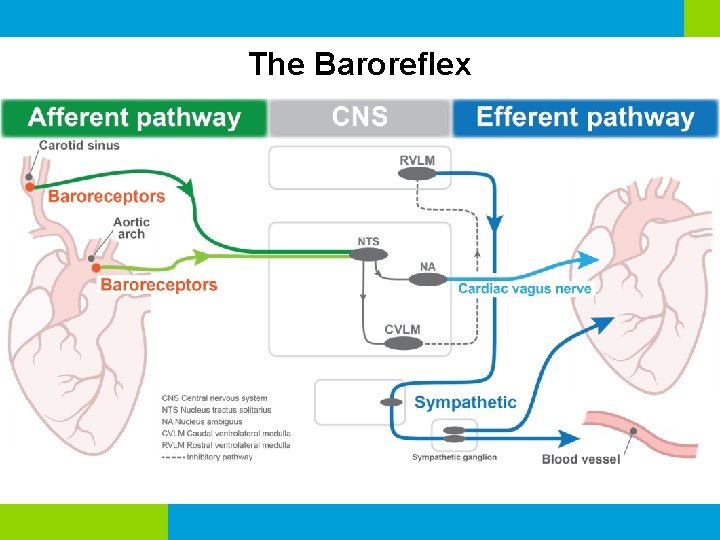
The Baroreflex
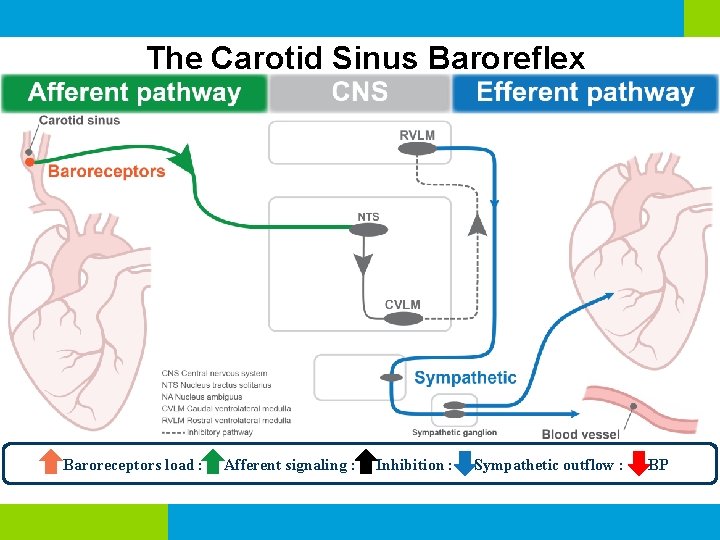
The Carotid Sinus Baroreflex Baroreceptors load : Afferent signaling : Inhibition : Sympathetic outflow : BP
Mobius. HD™: Baroreceptor Modulation 1. Mobius. HD is a passive implant designed to reshape the carotid sinus, delivered using standard percutaneous techniques and angiographic visualization 2. Mobius. HD is designed to exert just enough radial force on the vessel to reshape it in the diastolic phase, and prevent migration in the systolic phase 3. Reshaping the vessel increases the differential strain, and therefore the stretch, measured by the baroreceptors with every pulsatile wave, concentrated within the windows of the device
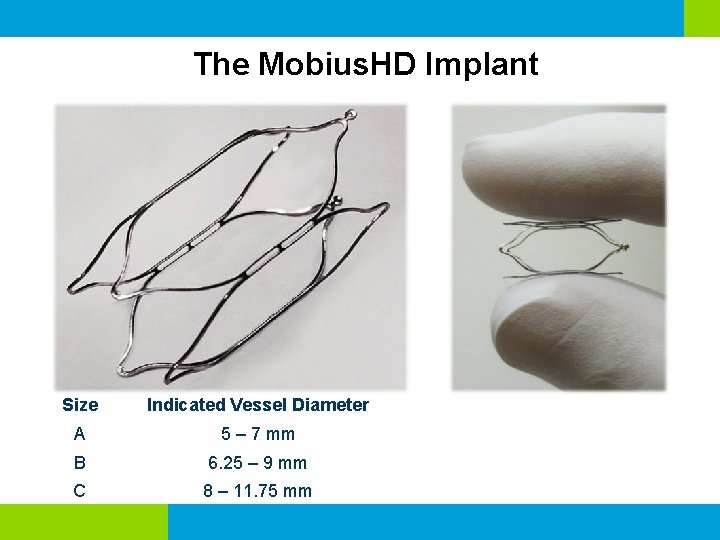
The Mobius. HD Implant Size Indicated Vessel Diameter A 5 – 7 mm B 6. 25 – 9 mm C 8 – 11. 75 mm
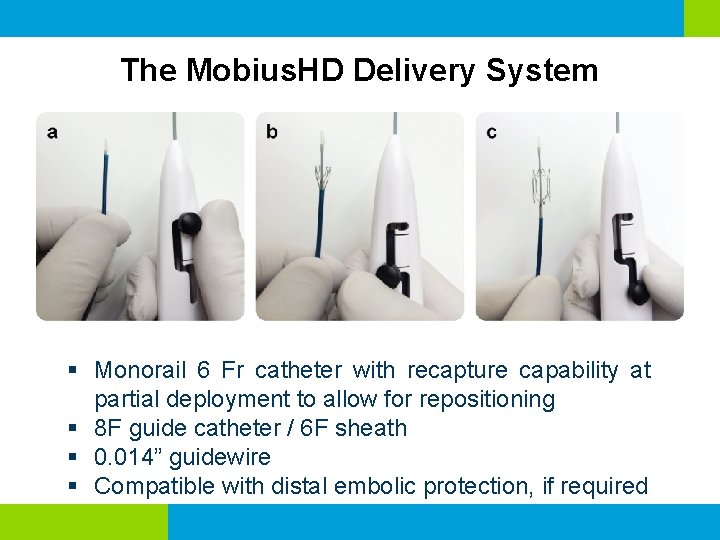
The Mobius. HD Delivery System § Monorail 6 Fr catheter with recapture capability at partial deployment to allow for repositioning § 8 F guide catheter / 6 F sheath § 0. 014” guidewire § Compatible with distal embolic protection, if required
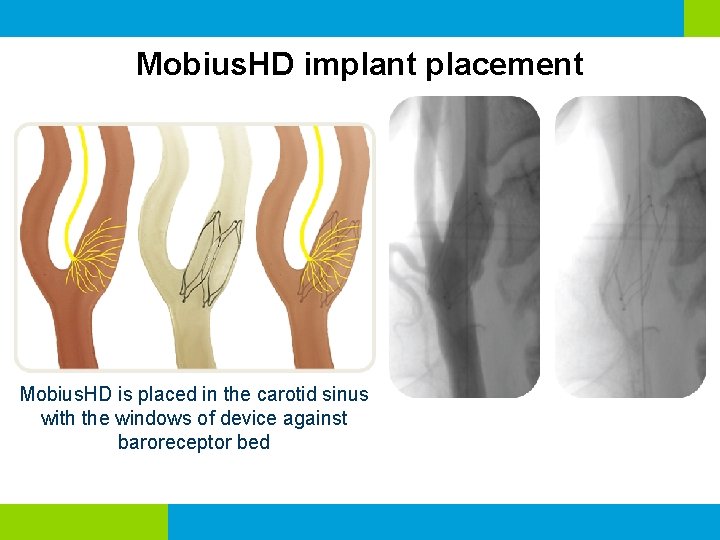
Mobius. HD implant placement Mobius. HD is placed in the carotid sinus with the windows of device against baroreceptor bed
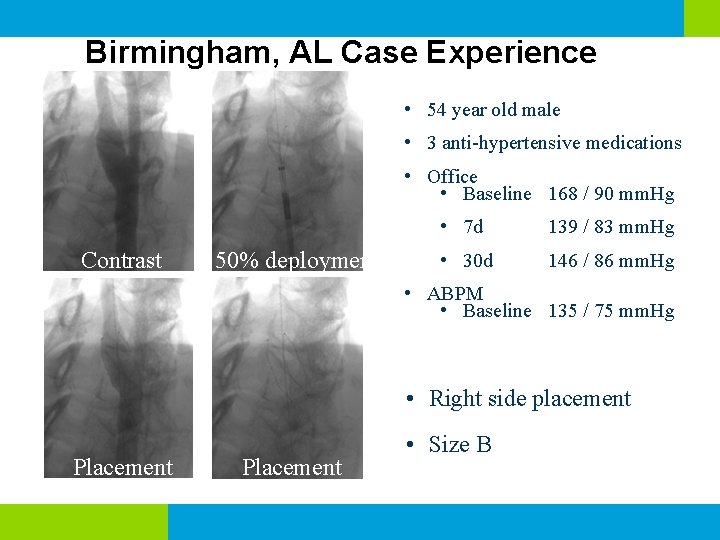
Birmingham, AL Case Experience • 54 year old male • 3 anti-hypertensive medications • Office • Baseline 168 / 90 mm. Hg Contrast 50% deployment • 7 d 139 / 83 mm. Hg • 30 d 146 / 86 mm. Hg • ABPM • Baseline 135 / 75 mm. Hg • Right side placement Placement • Size B

The CALM Clinical Studies Controlling and Lowering Blood Pressure using Mobius. HD: A Prospective Multicenter Safety Study • 20 patient safety study in the US – Concurrent 30 patient study in Europe • Patients with stage 2 resistant hypertension – >160 mm. Hg office cuff BP on ≥ 3 drugs
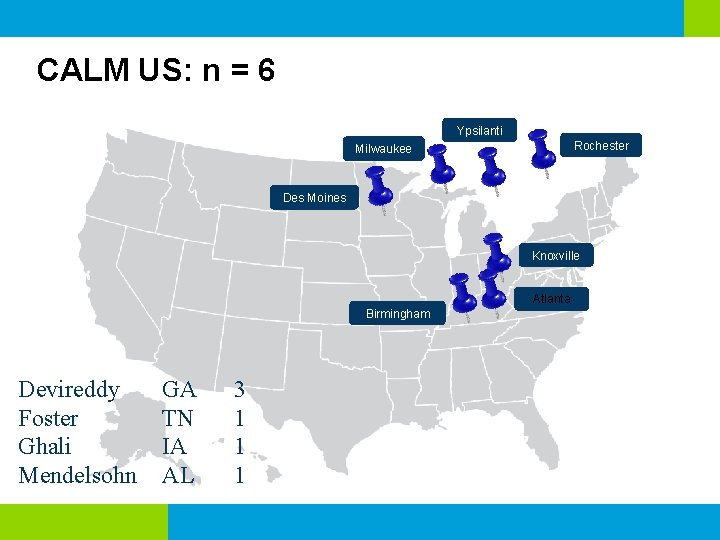
CALM US: n = 6 Ypsilanti Rochester Milwaukee Des Moines Knoxville Atlanta Birmingham Devireddy Foster Ghali Mendelsohn GA TN IA AL 3 1 1 1
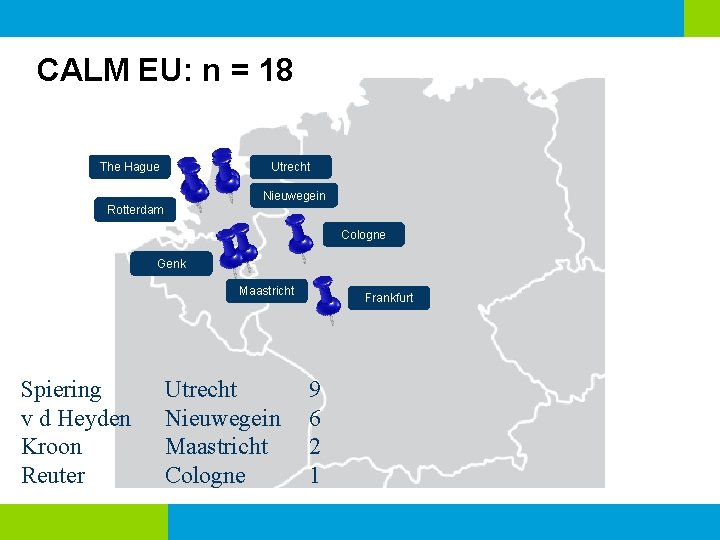
CALM EU: n = 18 The Hague Utrecht Nieuwegein Rotterdam Cologne Genk Maastricht Spiering v d Heyden Kroon Reuter Utrecht Nieuwegein Maastricht Cologne Frankfurt 9 6 2 1

CALM Study Safety, n = 24 • 12 patients have met the 6 month safety endpoint • 12 SAEs after treatment: o Procedure/device-related (n=2) o Hypotensive symptoms (n=4) o Uncontrolled hypertension (n=2) o Non study-related (n=4)
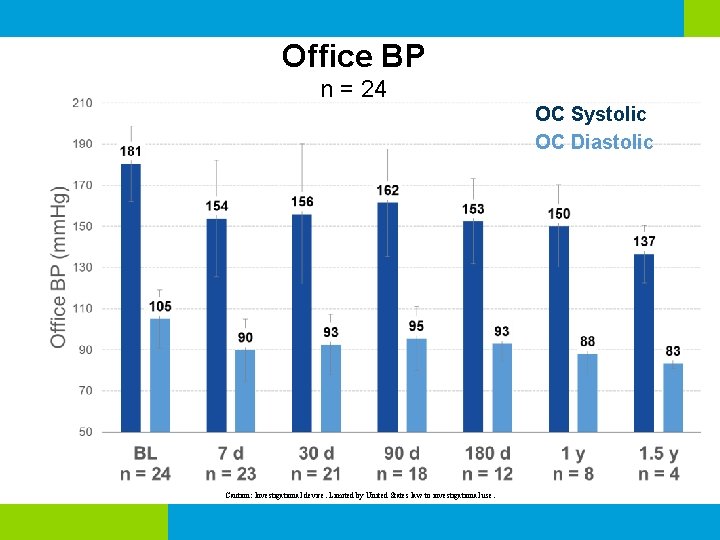
Office BP n = 24 OC Systolic OC Diastolic Caution: Investigational device. Limited by United States law to investigational use.
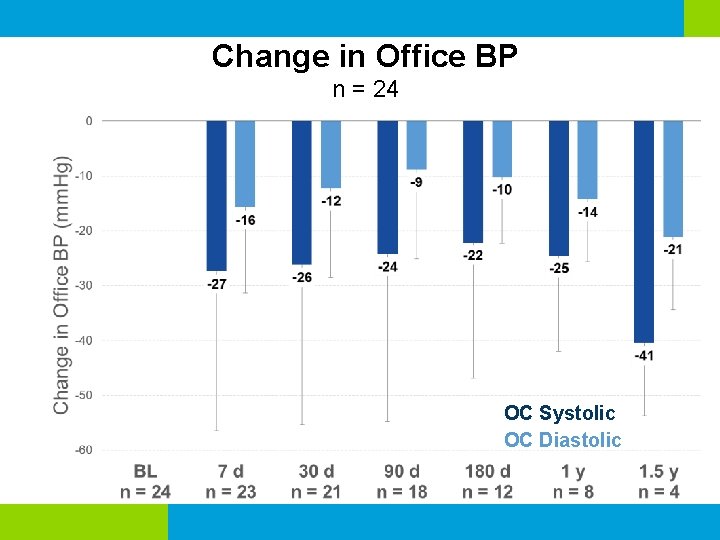
Change in Office BP n = 24 OC Systolic OC Diastolic
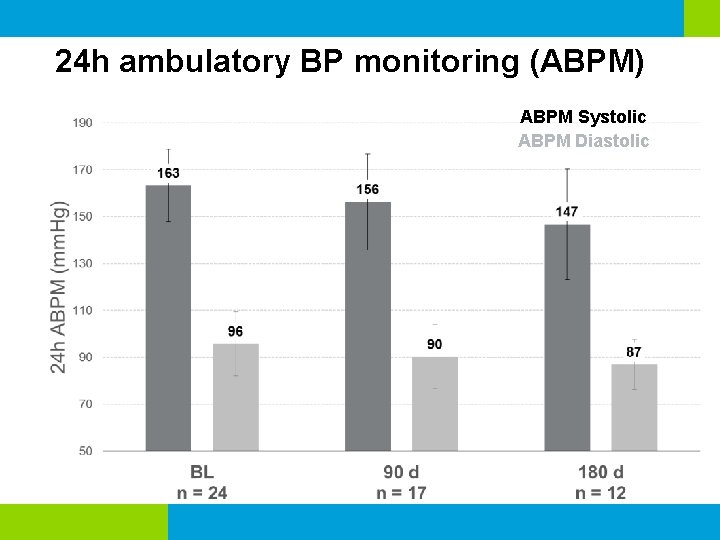
24 h ambulatory BP monitoring (ABPM) ABPM Systolic ABPM Diastolic
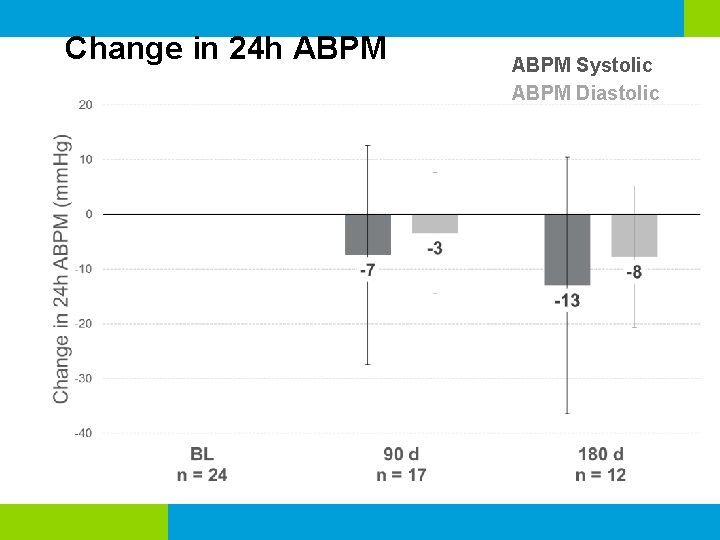
Change in 24 h ABPM Systolic ABPM Diastolic
Conclusion In this initial feasibility study, Mobius. HD appears safe and shows promising results in lowering blood pressure in patients with stage 2 resistant hypertension
- Slides: 18