MMVsupported projects Research Lead optimization Miniportfolio GSK Phenotypic
- Slides: 2

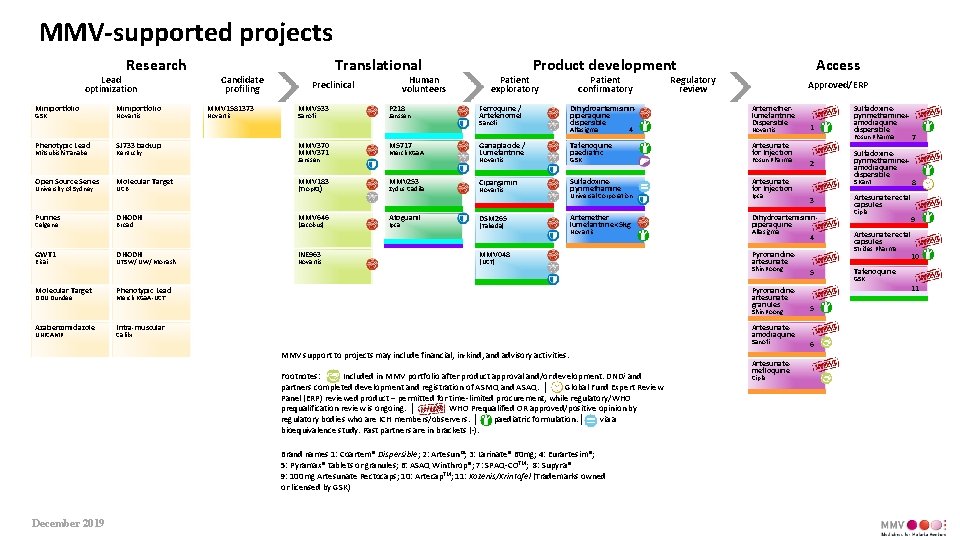
MMV-supported projects Research Lead optimization Miniportfolio GSK Phenotypic Lead Mitsubishi Tanabe Open Source Series University of Sydney Purines Celgene GWT 1 Eisai Molecular Target DDU Dundee Miniportfolio Novartis SJ 733 backup Kentucky Molecular Target UCB DHODH Broad DHODH UTSW/ UW/ Monash Candidate profiling MMV 1581373 Novartis Translational Human volunteers Preclinical MMV 533 Sanofi MMV 370 MMV 371 Janssen MMV 183 (Trop. IQ) MMV 646 (Jacobus) INE 963 Novartis P 218 Janssen M 5717 Merck KGa. A MMV 253 Zydus Cadila Atoguanil Ipca Product development Patient exploratory Ferroquine / Artefenomel Sanofi Ganaplacide / Lumefantrine Novartis Cipargamin Novartis DSM 265 (Takeda) Patient confirmatory Access Regulatory review Approved/ERP Dihydroartemisininpiperaquine dispersible 4 Alfasigma Artemetherlumefantrine Dispersible Tafenoquine paediatric Artesunate for Injection GSK Sulfadoxinepyrimethamine Universal Corporation Artemether lumefantrine <5 kg Novartis MMV 048 (UCT) Novartis Fosun Pharma Merck KGa. A-UCT Ipca Alfasigma UNICAMP Intra-muscular 4 Sanofi Footnotes: Included in MMV portfolio after product approval and/or development. DNDi and partners completed development and registration of ASMQ and ASAQ. │ Global Fund Expert Review Panel (ERP) reviewed product – permitted for time-limited procurement, while regulatory/WHO prequalification review is ongoing. │ WHO Prequalified OR approved/positive opinion by regulatory bodies who are ICH members/observers. │ paediatric formulation. │ via a bioequivalence study. Past partners are in brackets (-). Brand names 1: Coartem® Dispersible; 2: Artesun®; 3: Larinate® 60 mg; 4: Eurartesim®; 5: Pyramax® tablets or granules; 6: ASAQ Winthrop®; 7: SPAQ-COTM; 8: Supyra® 9: 100 mg Artesunate Rectocaps; 10: Artecap. TM; 11: Kozenis/Krintafel (Trademarks owned or licensed by GSK) Artesunatemefloquine Cipla Sulfadoxinepyrimethamine+ amodiaquine dispersible 5 8 Artesunate rectal capsules Cipla 9 Artesunate rectal capsules Strides Pharma 10 Tafenoquine GSK 11 5 Artesunateamodiaquine Calibr MMV support to projects may include financial, in-kind, and advisory activities. December 2019 3 Pyronaridineartesunate Shin Poong 7 Fosun Pharma S Kant Dihydroartemisininpiperaquine Shin Poong Azabenzimidazole 2 Artesunate for Injection Pyronaridineartesunate granules Phenotypic Lead 1 Sulfadoxinepyrimethamine+ amodiaquine dispersible 6

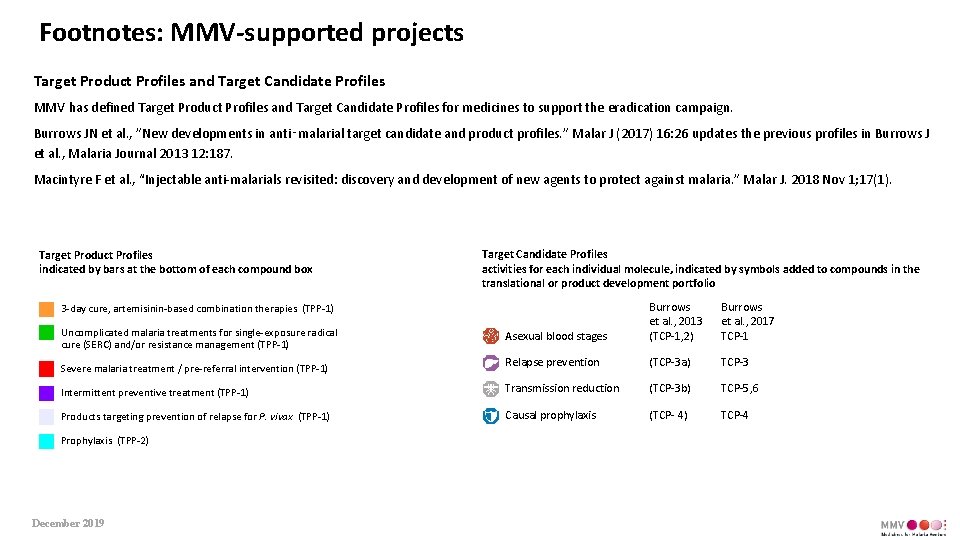
Footnotes: MMV-supported projects Target Product Profiles and Target Candidate Profiles MMV has defined Target Product Profiles and Target Candidate Profiles for medicines to support the eradication campaign. Burrows JN et al. , ”New developments in anti‑malarial target candidate and product profiles. ” Malar J (2017) 16: 26 updates the previous profiles in Burrows J et al. , Malaria Journal 2013 12: 187. Macintyre F et al. , “Injectable anti-malarials revisited: discovery and development of new agents to protect against malaria. ” Malar J. 2018 Nov 1; 17(1). Target Product Profiles indicated by bars at the bottom of each compound box Target Candidate Profiles activities for each individual molecule, indicated by symbols added to compounds in the translational or product development portfolio Asexual blood stages Burrows et al. , 2013 (TCP-1, 2) Burrows et al. , 2017 TCP-1 Relapse prevention (TCP-3 a) TCP-3 Intermittent preventive treatment (TPP-1) Transmission reduction (TCP-3 b) TCP-5, 6 Products targeting prevention of relapse for P. vivax (TPP-1) Causal prophylaxis (TCP- 4) TCP-4 3 -day cure, artemisinin-based combination therapies (TPP-1) Uncomplicated malaria treatments for single-exposure radical cure (SERC) and/or resistance management (TPP-1) Severe malaria treatment / pre-referral intervention (TPP-1) Prophylaxis (TPP-2) December 2019