MLT 242 Clinical Chemistry II Hemoglobin Production Disorders

MLT 242: Clinical Chemistry II Hemoglobin Production Disorders

Overview of Iron p. 405 -407 • Essential mineral to most living organisms • Most abundant trace element • 2 -2. 5 of the 3 -5 grams of iron in our bodies is found in hemoglobin (RBCs and RBC precursors) – Myoglobin: oxygen-carrying molecule of muscle – Tissue: bound to enzymes – Bone marrow, spleen, liver: storage forms

Where does iron come from? • Two types • Heme • meats, especially organ meats • Non-Heme • spinach, beets, beans, almonds, bran flakes. . etc • Typical dietary intake is 10 -20 mg per day.
Forms of Iron • Ferrous(Fe 2+) – Absorbed form • Ferric (Fe 3+) – Transport and storage form – Delivered to cells having receptor sites • Gut mucosal cells • Liver cells • RE system cells

Regulation • Absorption: intestines – must be in Fe 2+ state • Iron “stores”: liver, spleen, bone marrow – Fe 3+
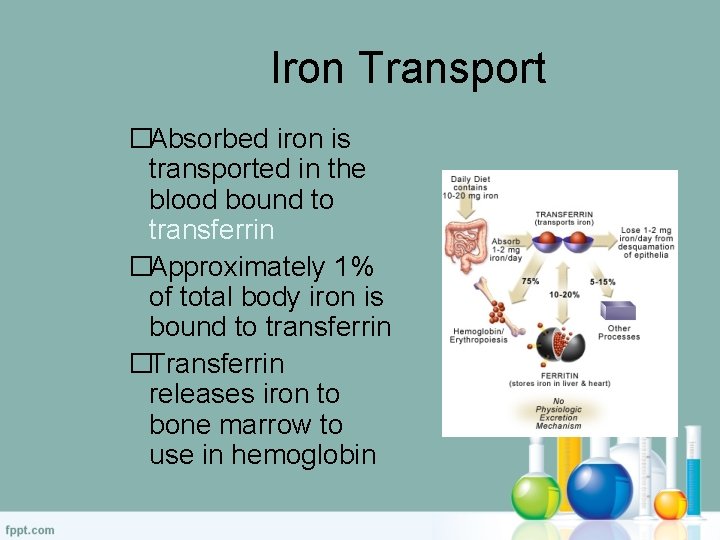
Iron Transport �Absorbed iron is transported in the blood bound to transferrin �Approximately 1% of total body iron is bound to transferrin �Transferrin releases iron to bone marrow to use in hemoglobin

Functions of Iron • Essential element of heme and hemoglobin • Component of methemoglobin, myoglobin and some enzymes • Cellular oxidative mechanisms

Hemoglobin • Structure, Synthesis, Degradation and Role – Refer to Hematology notes for review
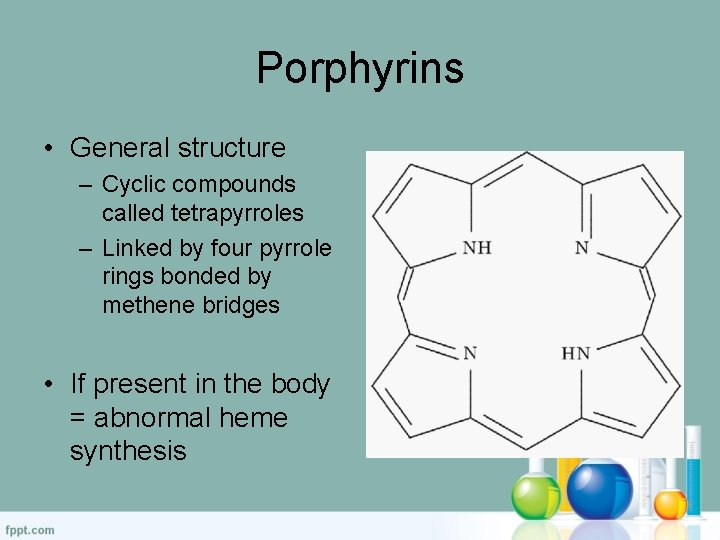
Porphyrins • General structure – Cyclic compounds called tetrapyrroles – Linked by four pyrrole rings bonded by methene bridges • If present in the body = abnormal heme synthesis
Physical properties of Porphyrins • Color – Red-violet to red-brown in color • Fluorescence – Red around 400 nm • Chelation – Chelate metal atoms (Fe)
Porphyrin Synthesis & Control • Synthesis – Bone marrow and liver are the main site – Synthesis occurs in mitochrondria and cytoplasm of cell • Control – Enzyme: δ-aminolevulinic acid (ALA) • Found in liver – Negative Feedback Mechanism – Rate of heme syntheis is flexible and can change rapidly in response to external stimuli

Porphyrins: Clinically Significant • Uroporphyrin: URO – urine • Coproporphyrin: COPRO – Both depending on p. H of urine • Protoporphyrin: PROTO – feces
Porphyrinogens • Reduced form of porphyrins • Functional precursor of heme • Difficult to measure due to instability and colorlessness – Do not fluoresce

Myoglobin • Heme protein found in skeletal and cardiac muscle • Unable to release oxygen, except under low oxygen tension • Main function: transport oxygen from muscle cell membrane to mitochondria • Serves as an extra reserve of oxygen to help exercising muscle maintain activity longer • Used to diagnose acute myocardial infarction

Lead • Found in the environment and in paint • Considered a toxin, plays no known role in NORMAL human physiology • Exposure primarily respiratory or gastrointestinal • Half-life in whole blood= 2 -3 weeks – Half-life= the time required by the body, tissue or organ to metabolize or inactivate half the amount of substance taken in

Lead • Absorption – Depends on age, nutritional status and other substances that are present • Transport – Once in the blood, 94% transferred to RBC bound to hgb – Once it reaches its half-life, lead is distributed to soft tissues, such as kidneys, liver and brain. Final storage is in soft tissue(5%) and bone (95%) • Excretion – Urine (76%) – Feces (16%) – Other (8%)

Hemoglobin Disorders • Iron Deficiency • Hemosiderosis • Hemochromatosis – See chart on pg 406 – See also Hematology notes • Porphyrias – Due to enzyme deficiencies – Overproduction of heme precursors

Specimen Requirements for Iron Studies – Serum without anticoagulant – Plasma with heparin – Oxalate, citrate or EDTA binds Fe ions, so they are unacceptable – Early morning sample preferred due to diurnal variation – No hemolysis
Iron Studies Profile – Total Iron ( serum ) – TIBC – % Iron Saturation ( Fe Sat ) • (Total Iron/TIBC) x 100

Direct Measurement – Iron – Men: 65 -165 µg/d. L – Women: 45 -160 µg/d. L – Transferrin • 200 -360 mg/d. L – Ferritin • Male: 20 -250 ng/m. L • Female: 10 -120 ng/m. L
Iron Measurement • Measured by colorimetric Procedure • Separate Fe from transferrin with strong acid • Reduced from Ferric to ferrous • Add chromogen (measured)

Iron • Increased absorption • Hemolytic anemia • Lead poisoning • Pernicious anemia • Megaloblastic anemia • Hepatitis • Decreased intake • Increased need • Increased loss
Indirect Measurement – TIBC (Total iron-binding capacity) • Measured by the Pre-treatment and Colorimetric Method • Add ferric Fe to saturate binding sites • Mg. CO 3 is added to remove unbound ferric Fe • Mixture is centrifuged and supernatant testing using serum Fe methodology • Normal Value: 250 -425 µg/d. L
TIBC • Increased –Late pregnancy –IDA –Following hemorrhage –Following destruction of liver cells • Decreased –Decreased synthesis of transferrin –Increased loss of urine proteins

Lab Methods for Porphyrias • Watson-Schwartz for Urinary PBG (porphobilinogen) – Screen for acute intermittent porphyria – Specimen: Urine • Qualitative: 1 st morning • Quantitative: 24 hour – Reference Range • <2 mg/day
Lab Methods for Myoglobin • Immunoassays – Chemiluminescence, fluorescence, immunochromatic • Specimen requirements – Usually plasma
Specimen Requirements: Lead • Whole blood – Why? Circulating lead found in the RBC • Royal blue top with EDTA anticoagulant • EDTA – Lead-free containers – Venous sample preferred • Capillary specimens can be used for screening • Urine

Lab Methods for Lead • Atomic Absorption Spectrophotometry: directly on lead chip • Anodic stripping voltammetry

Where does iron come from? • Heme: meats, especially organ meats • Non-heme: spinach, beets, beans, almonds, bran flakes, etc.

forms • Absorbed in intestines- absorbed form: Ferrous • Transported to or stored in the liver, spleen, bone moarrow- transport/storage form: Ferric
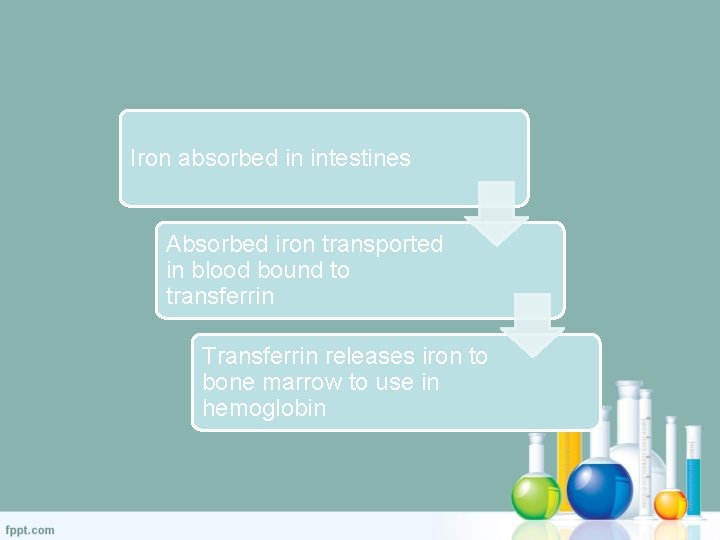
Iron absorbed in intestines Absorbed iron transported in blood bound to transferrin Transferrin releases iron to bone marrow to use in hemoglobin
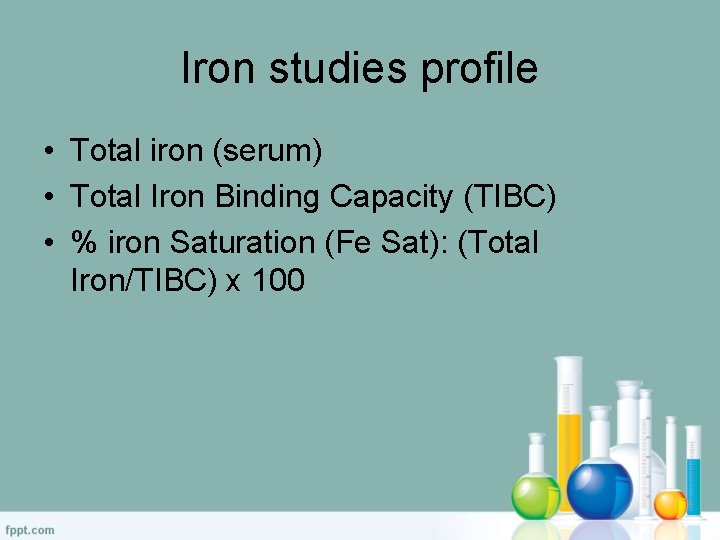
Iron studies profile • Total iron (serum) • Total Iron Binding Capacity (TIBC) • % iron Saturation (Fe Sat): (Total Iron/TIBC) x 100

Specimen requirements • Serum without anticoagulant • Plasma with heparin • No hemolysis
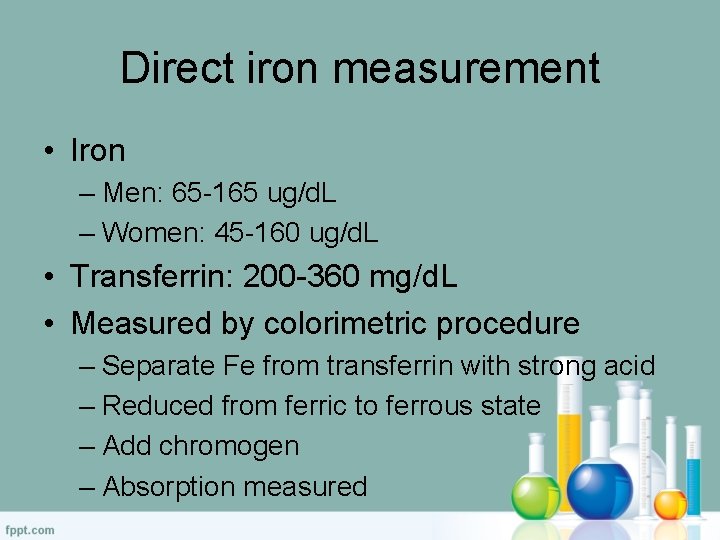
Direct iron measurement • Iron – Men: 65 -165 ug/d. L – Women: 45 -160 ug/d. L • Transferrin: 200 -360 mg/d. L • Measured by colorimetric procedure – Separate Fe from transferrin with strong acid – Reduced from ferric to ferrous state – Add chromogen – Absorption measured
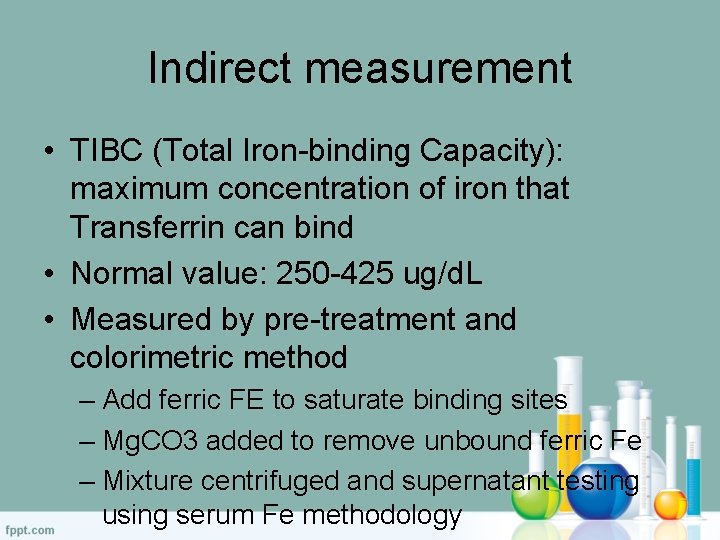
Indirect measurement • TIBC (Total Iron-binding Capacity): maximum concentration of iron that Transferrin can bind • Normal value: 250 -425 ug/d. L • Measured by pre-treatment and colorimetric method – Add ferric FE to saturate binding sites – Mg. CO 3 added to remove unbound ferric Fe – Mixture centrifuged and supernatant testing using serum Fe methodology
POINte measurement of tibc • Pointe analyzer uses Fe value and UIBC value (unsaturated iron binding capacity) to calculate TIBC • Total Iron Binding Capacity (max concentration of iron that can be bound to transferrin) – serum FE level = UIBC (reserve iron binding capacity; not being used) – Serum Fe + UIBC = TIBC • UIBC normal range: 150 -375 ug/d. L

Increased/ decreased iron • Increased – – – Increased adsorption Hemolytic anemia Lead poisoning Pernicious anemia Megaloblastic anemia Hepatitis • Decreased – – IDA Decreased intake Increased need Increased loss

Increased/decreased tibc • Increased – – – Late pregnancy IDA Oral contraceptives Following hemorrhage Following destruction of liver cells • Decreased – Decreased synthesis of transferrin – Increased loss of urine proteins (nephrotic syndrome or kidney disease) – Hemochromatosis – Malnutrition

MLT 242: Clinical Chemistry II Toxicology and TDM

Toxicology • Study of poisons • Clinical: toxin exposure to disease – Intentional – Accidental – Homicide – Occupational

Toxicity • Acute • Chronic

Alcohols • Different classes – Ex. Methanol, isopropanol, ethylene glycol • Symptoms of CNS depressant – Disorientation – Slurred speech – Confusion – Euphoria – Paralysis
Ethanol • Converted to acetalaldehyde • Chronic exposure = liver damage • Detection – Coupled reaction: NADH measured from oxidation of acetaldehyde by the enzyme alcohol dehydrogenase • Other lab tests – GGT, AST increased

Ethylene Glycol • • Found in antifreeze and hydraulic fluid Sweet tasting Metabolite: glycolic acid Complications – CNS, metabolic acidosis, acute renal failure • Detection – Wood’s lamp – GC • Other lab tests – Osmolal gap, UA with microscopic, blood gases, anion gap, BUN, Creat

Carbon Monoxide • Colorless, odorless, tasteless gas • By product of incomplete combustion of carbon containing substances • Action: binds to Hgb and blocks oxygenation causing hypoxia • Methods of testing – GC: reference – Cooximetry

Salicylates (NSAID) • Asprin • Interferes with platelet aggregation by decreasing thromboxane and prostaglandin formation • Overdose: – Acid-base imbalance

Acetaminophen • Overdose – Hepatotoxicity • Toxic metabolite N-acetyl-p-benzoquinoneimine • If glutathione depletes metabolite then accumulates • Methods – enzymatic • Arylacyamide amindohydrolase hydrolyzes acetaminophen to p-aminophenol and acetate – HPLC – Immunoassay

Drugs of Abuse • Qualitative screening = urine; if positive confirmation necessary • Detects recent use • Panel – – – – Amphetamines Barbituates Benzodiazepines Canabinoids Cocaine Opiates Phencyclidine Tricyclic anitdepressants
Amphetamines • Stimulants • Compounds – Methamphetamine – Pseudophedrine – Ecstasy • Overdose – Hypertension – Convulsions – Cardiac arrthymia
Cannabinoids • THC (tetrahydrocannabinol) • Marijuana • Effects – CNS: relaxation, heightened sensory awareness – Impair short term memory – Increased appetite

Cocaine • CNS stimulator and local anesthetic • Absorbed by many routes – Nasal inhalation – IV – Smoked = crack

Opiates • Derived from opium poppy – Opium – Heroin – Morphine – Codeine – Methadone
Phencyclidine • Hallucinogen – LSD – PCP

Benzodiazepines • CNS depressants • Valium, ativan
Therapeutic Drug Monitoring • Half-life – Time it takes for ½ of the dose to be eliminated from the bloodstream • Steady state – Takes about 4 -7 doses of the medication
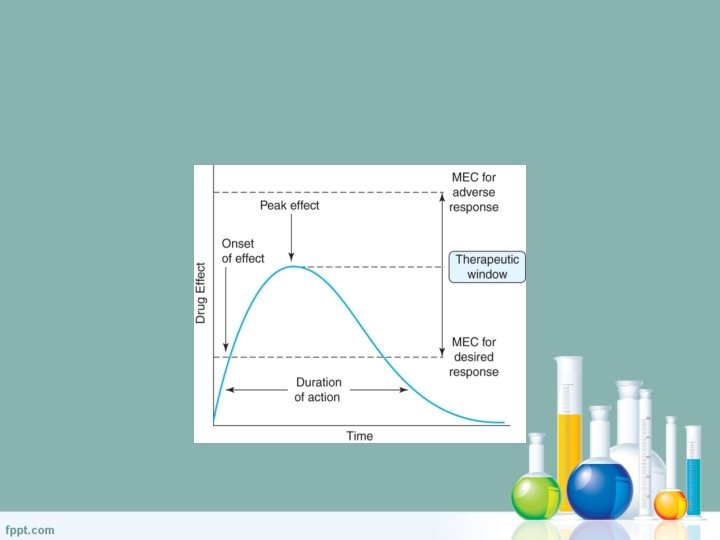
Therapeutic Drug Monitoring • Why? – Dosage = maximum therapeutic effect – Minimal adverse effects – Appropriate concentration

Drugs to be Monitored • • Cardioactive: digoxin Antibiotics: Vancomycin, Gentamycin Antiepileptic: phenytoin, phenobarbital Psychoactive: lithium Antiasthmatic: theophyilline Immunosuppressive: cyclosporine Antineoplastics: methotrexate
- Slides: 58