MLS 202 HAEMOGLOBIN ESTIMATION METHODS AKINBO D B

MLS 202 HAEMOGLOBIN ESTIMATION METHODS AKINBO D. B. Lecture Series
Haemoglobin • Haemoglobin (Hb) is the main component of RBCs and accounts for approximately 34% of the RBCs by weight, having a molecular weight of 64. 5 KDa. • Haemoglobin is a chromoprotein containing two pairs of polypeptide chains α 2 β 2 and four haem groups each having an atom of ferrous iron(Fe+2). • Each haemoglobin molecule carries four molecule of oxygen and each gram of haemoglobin carry 1. 34 ml of oxygen. • Iron content of Hb is 0. 347 gm/100 g.
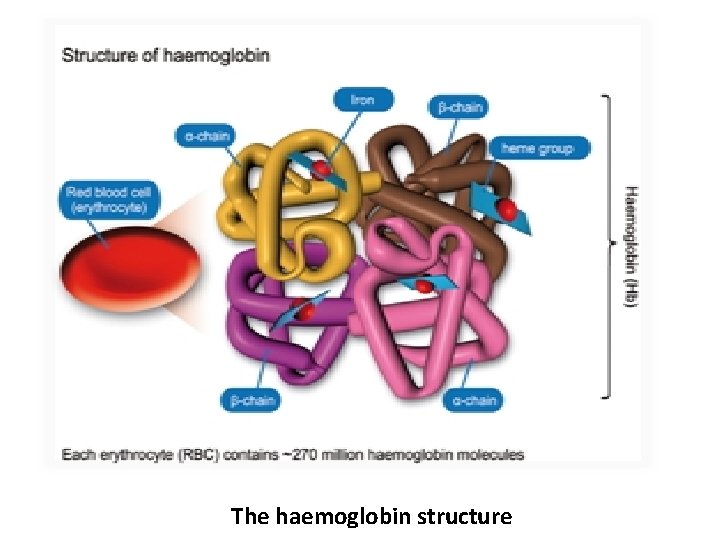
The haemoglobin structure
Forms of Haemoglobin i. Oxyhaemoglobin (Hb O 2): This forms from the combination of normal haemoglobin molecule with oxygen molecule and formed in the red blood cells as they take up oxygen in the lungs. ii. Carboxyhaemoglobin (Hb CO): It forms from the exposure of normal haemoglobin to carbon dioxide or carbonmonoxide and imparts a cherry red coloration to blood and skin in high concentrations. It is reversible
Forms of Haemoglobin iii. Sulfhaemoglobin (SHb): This forms when sulphur combines with the haeme group of haemoglobin by the action of certain drugs and chemicals such as sulphonamides and presence of sulphur in the air. It is green in colour, unable to carry oxygen and once formed, is irreversible and remains in the carrier RBC. iv. Methaemoglobin: The iron in this haemoglobin is in the ferric state and incapable of reversibly combining with oxygen. It has a dark brown colour with a normal concentration of 1 -2%.
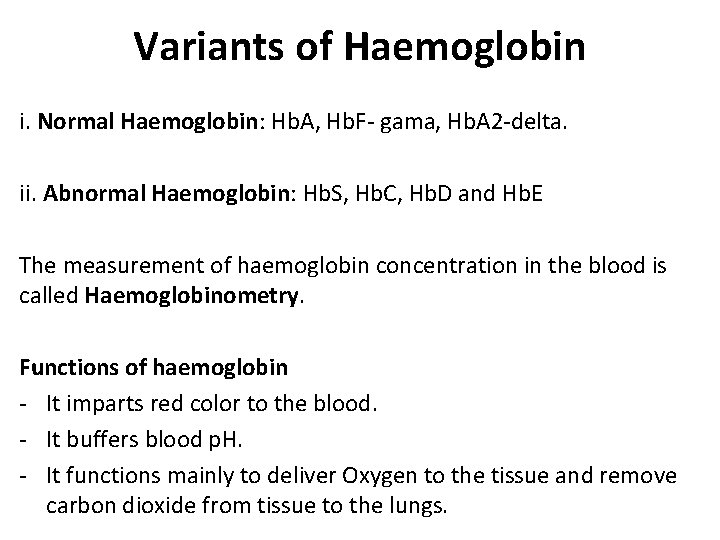
Variants of Haemoglobin i. Normal Haemoglobin: Hb. A, Hb. F- gama, Hb. A 2 -delta. ii. Abnormal Haemoglobin: Hb. S, Hb. C, Hb. D and Hb. E The measurement of haemoglobin concentration in the blood is called Haemoglobinometry. Functions of haemoglobin - It imparts red color to the blood. - It buffers blood p. H. - It functions mainly to deliver Oxygen to the tissue and remove carbon dioxide from tissue to the lungs.
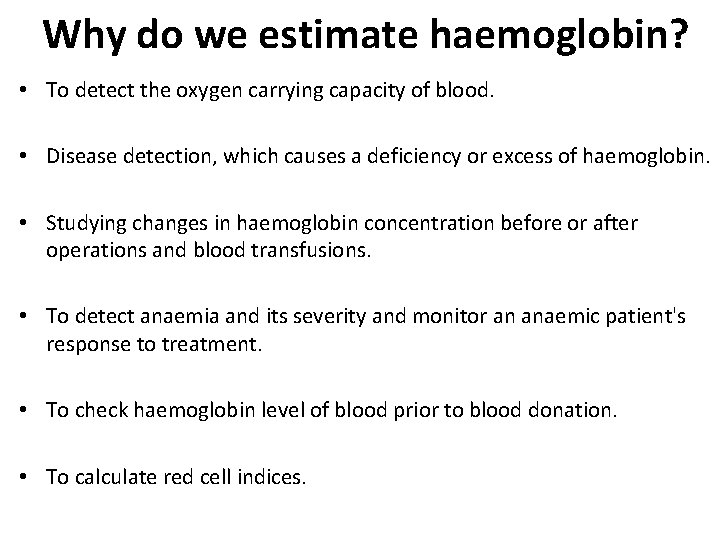
Why do we estimate haemoglobin? • To detect the oxygen carrying capacity of blood. • Disease detection, which causes a deficiency or excess of haemoglobin. • Studying changes in haemoglobin concentration before or after operations and blood transfusions. • To detect anaemia and its severity and monitor an anaemic patient's response to treatment. • To check haemoglobin level of blood prior to blood donation. • To calculate red cell indices.
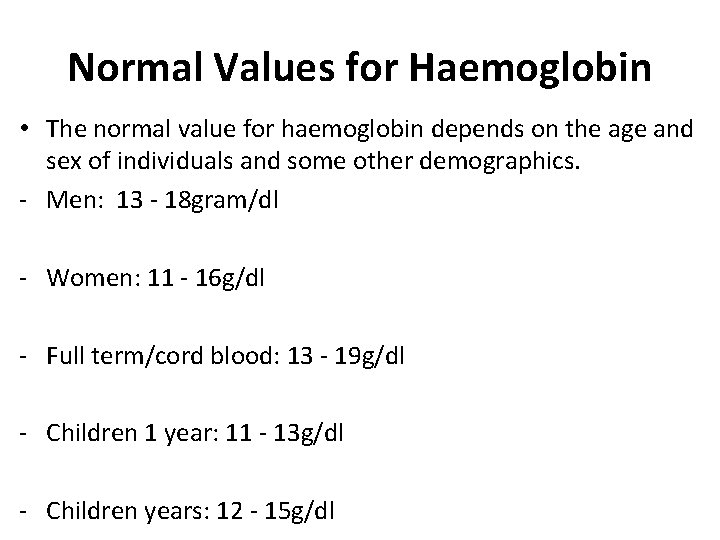
Normal Values for Haemoglobin • The normal value for haemoglobin depends on the age and sex of individuals and some other demographics. - Men: 13 - 18 gram/dl - Women: 11 - 16 g/dl - Full term/cord blood: 13 - 19 g/dl - Children 1 year: 11 - 13 g/dl - Children years: 12 - 15 g/dl

Required samples for Hb estimation • Capillary blood from finger prick. • Intravenous sample: Should be well anticoagulated, preferably in EDTA. Liquid anticoagulants should not be used as they dilute and decrease Hb concentration.
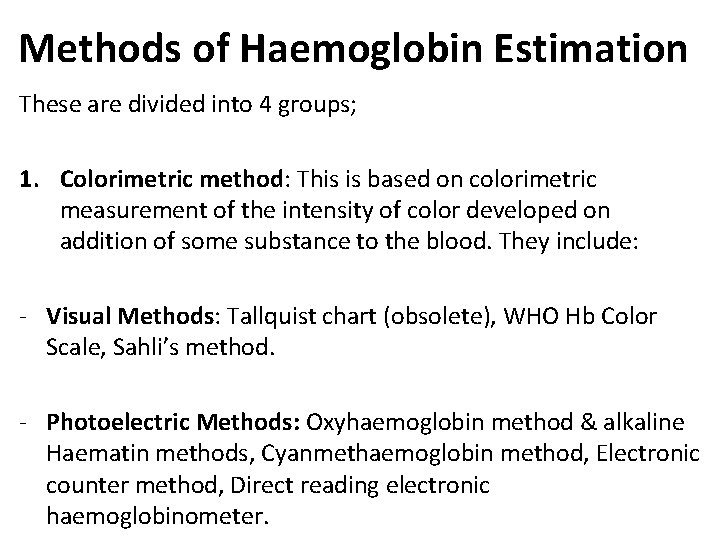
Methods of Haemoglobin Estimation These are divided into 4 groups; 1. Colorimetric method: This is based on colorimetric measurement of the intensity of color developed on addition of some substance to the blood. They include: - Visual Methods: Tallquist chart (obsolete), WHO Hb Color Scale, Sahli’s method. - Photoelectric Methods: Oxyhaemoglobin method & alkaline Haematin methods, Cyanmethaemoglobin method, Electronic counter method, Direct reading electronic haemoglobinometer.
Methods of Haemoglobin Estimation 2. Gasometric Method or Measurement of O 2 carrying capacity of Hb: It measures the O 2 -carrying capacity of Hb and can not be used for mass screening but used in referral or research laboratories only. 3. Chemical Method or Measurement of iron content of Hb: This measures the iron content of Hb and used only for research purpose 4. Specific gravity method: It is a very rapid method and is useful for screening blood donors for aneamia in blood donation program especially in resource-limited settings. The normal specific gravity of blood ranges from 1. 048 -1. 066.
Commonly Used Visual Haemoglobin Estimation Methods i. The WHO Haemoglobin Color Scale: This technique of estimating haemoglobin involves placing a drop of blood on a particular type of chromatography paper, and the color developed after absorption compared against a printed scale of colour. • This color corresponds to different levels of haemoglobin ranging from 4 -14 g/dl which forms a color scale. • It is rapid, simple, inexpensive and reliable.
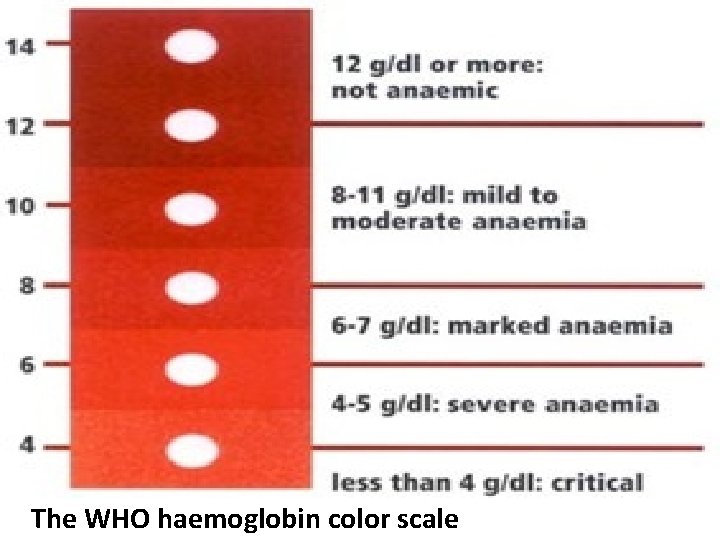
The WHO haemoglobin color scale
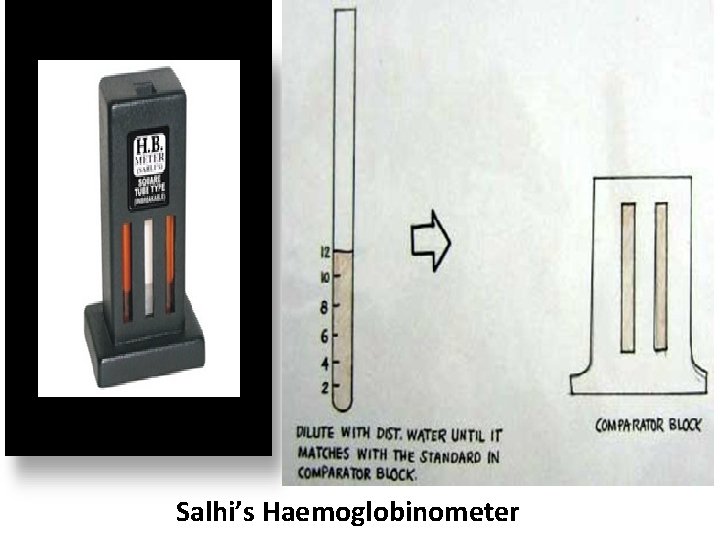
Commonly Used Visual Haemoglobin Estimation Methods ii. Sahli’s Method or Acid Hematin method Principle: Haemoglobin is converted to acid haematin by N/10 HCl and the resulting brown colour is compared with standard brown glass reference blocks. The intensity of the brown colour depends on the amount of acid haematin produced, and this is directly proportional to the amount of haemoglobin in the blood sample.
Sahli’s Method Procedure • Place N/10 HCl into the Sahli’s Hb tube up to the lowest mark. • Deliver 20μl (0. 02 ml) of blood from a Hb pipette into the tube thereafter. • Stir with a glass rod/stirrer and wait for 10 minutes to allow color development. • Add distilled water drop by drop and stir till color matches with the comparator because 95% of Hb is converted at the end of 10 mins and others much later. • Take the reading at upper meniscus.
Salhi’s Haemoglobinometer
Sahli’s Method Advantages - Simple bedside test. - Reagents and apparatus are cheap. Disadvantages - There can be visual error. - Carboxy-, met- and sulfhaemoglobins cannot be converted to acid hematin. - Comparator can fade over the years. - Color attainment of acid hematin takes long time and also fades quickly. - Source of light (day light or artificial) influences the color comparison.
Commonly Used Spectrophotometric Haemoglobin Estimation Methods i. Oxyhaemoglobin method • Principle: Blood is diluted in weak alkali (0. 04% ammoniun hydroxide, sp gravity: 0. 88) which lyses the red blood cells and release oxyhaemoglobin into the solution. This conversion is complete and immediate with a resulting stable colour. • In this method blood sample is mixed with a weak ammonia solution and the absorbance of this solution is measured in a spectrophotometer at 540 nm.
Commonly Used Spectrophotometric Haemoglobin Estimation Methods • Absorbance of this solution is compared with that of the standard solution. • It is a rapid & simple method, however derivatives other than oxy. Hb are not measured. Advantages - It is fast and no time is required for colour development. - It is a simple, economical and accurate method than visual comparative method (error of 2 -3%). Disadvantages - Standard solution not easily available and unstable. - Methhaemoglobin and carboxyhaemoglobin are not accurately detected.
Commonly Used Spectrophotometric Haemoglobin Estimation Methods • Cyanmethaemoglobin method: It is the preferred and most accurate method of determining the haemoglobin concentration in the haematology laboratory. • Principle: Blood is diluted in a solution of potassium ferricyanide and potassium cyanide. The ferricyanide oxidizes haemoglobin to methaemoglobin and potassium cyanide provides cyanide ions (CN–) to form Cyanmethaemoglobin. • The absorbance of the solution is then measured in a spectrophotometer at a wavelength of 540 nm or in a colorimeter using a yellow-green filter.
Cyanmethaemoglobin method Procedure • Take 5 ml of Drabkin’s solution in a large sized test tube. • Add 20 microlitres (20 ul) of well mixed anticoagulated venous blood, rinse the pipette and mix well. • Allow it to stand at room temperature for minutes. • Absorbance is measured against reagent blank at 540 nm either in a spectrophotometer or in colorimeter. Haemoglobin is derived from the formula: • Hb in g/d. L= Absorbance of test/Absorbance of Standard X Dilution Factor/100
Cyanmethaemoglobin method Advantages - All forms of Hb except SHb are readily converted to Hi. CN. - Direct comparison with Hi. CN standard possible. - Stability of the diluted sample and standard - Easy to perform the test and reagents are readily available. Disadvantages - Increased absorbance not due to haemoglobin may be caused by turbidity due to abnormal plasma proteins, hyperlipaemia, high WBC count or fat droplets. - Potassium cyanide in the solutions is poisonous, though it is present only in a very low concentration hence the reagents should be handled carefully. - Reagents and samples should be disposed along with the running water in the sink.
Specific gravity method (Physical method) • Haemoglobin as the largest single constituent, affects the specific gravity of blood more than other substances. • This procedure does not give the exact Hb value, as it is not accurate. It uses the principle that when a drop of whole blood is dropped into a solution of copper sulphate (Cu. So 4), which has a given specific gravity, the blood will maintain its own density for approximately 15 seconds. • The density of the drop is directly proportional to the amount of haemoglobin in that drop. If the blood is denser than the specific gravity of the solution, the drop sinks to the bottom, if not it will float.

Specific gravity method procedure • A drop of blood is allowed to fall in copper sulphate (Cu. So 4) solution of specific gravity 1. 053 from a height of 1 cm and is equivalent to Hb of 12. 5 grams. • This drop of blood is covered by Cu. So 4 and remains discrete for 15 -20 seconds. • If drop sinks within this time its specific gravity is higher but if it floats, the specific gravity is low hence low Hb. • Since the exact Hb value of the donor is not needed, the blood bank sets a cut-off value for men and women using Cu. So 4 solutions with corresponding specific gravity.
- Slides: 24