MLO 130 Hematology I Hemoglobin Red Cell Composition
MLO 130: Hematology I Hemoglobin

Red Cell Composition • Heme – 4 iron molecules surrounded by protoporphyrin ring • Globin – 2 pairs of globin chains made up of amino acids – Alpha and beta chains • 2, 3 -Diphosphoglycerate (2, 3 -D P G)
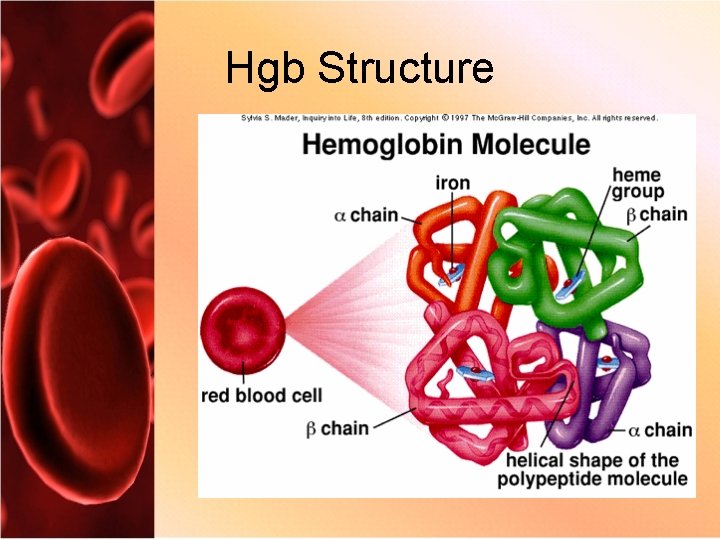
Hgb Structure
Hemoglobin Synthesis • The function of the RBC’s is to produce, package, protect, and transport hemoglobin among various tissues • Hemoglobin – Responsible for carrying oxygen (oxyhemoglobin) – Pulls CO 2 away from tissues • Keep balanced p. H between the blood
Genetics of Hgb • 3 types of hemoglobin in red cell production – Embryonic hemoglobin – Fetal hemoglobin – Adult hemoglobin • Chromosome 11 – Genes for epsilon, beta, gamma G and A, delta chains • Chromosome 16 – Alpha and zeta genes
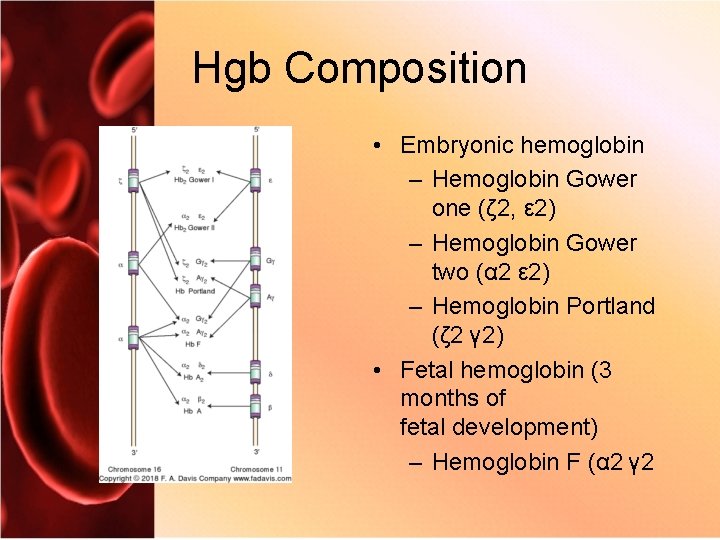
Hgb Composition • Embryonic hemoglobin – Hemoglobin Gower one (ζ 2, ε 2) – Hemoglobin Gower two (α 2 ε 2) – Hemoglobin Portland (ζ 2 γ 2) • Fetal hemoglobin (3 months of fetal development) – Hemoglobin F (α 2 γ 2

Hgb Composition • Adult hemoglobin (3 to 6 months after delivery) – Hemoglobin A (α 2 β 2): 95% to 98% – Hemoglobin A 2 (α 2 δ 2): 3% to 5% – Hemoglobin F (α 2 γ 2): less than 2%

Globin Chain Synthesis
Synthesis of Hemoglobin • Occurs in mitochondria and cytoplasm – Starts in pronormoblast stage – Ends in polychromatic erythrocyte in circulation – No ribosomes (RNA), no hgb production • Normal synthesis has 3 requirements – iron supply & delivery: transferrin – protoporphyrins – globin chains

Heme Synthesis
Hgb Function • Bind oxygen in lungs and transport to tissues • Requirements: – High oxygen affinity (1 molecule hgb binds 4 molecules of oxygen because of 4 heme Fe’s) – Unload oxygen • Dependent on 2, 3 -DPG • Oxygenated • Deoxygenated
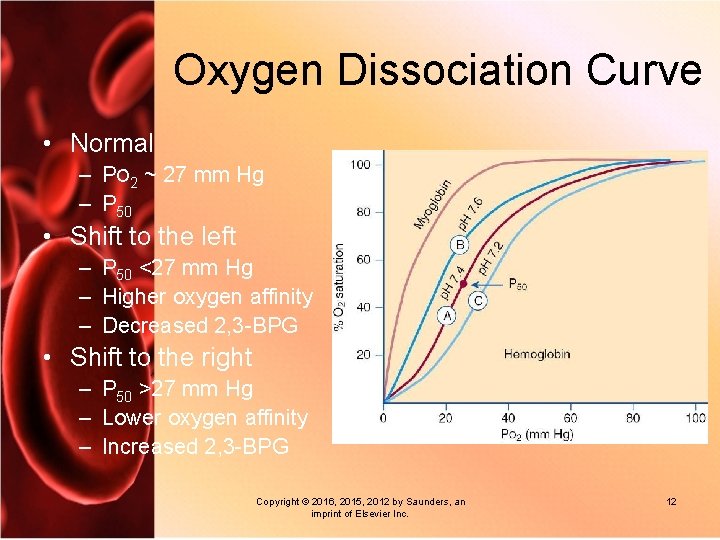
Oxygen Dissociation Curve • Normal – PO 2 ~ 27 mm Hg – P 50 • Shift to the left – P 50 <27 mm Hg – Higher oxygen affinity – Decreased 2, 3 -BPG • Shift to the right – P 50 >27 mm Hg – Lower oxygen affinity – Increased 2, 3 -BPG Copyright © 2016, 2015, 2012 by Saunders, an imprint of Elsevier Inc. 12
Abnormal Hemoglobins • Carboxyhemoglobin – High affinity for carbon monoxide (CO) – Increased in smokers and certain industrial workers • Methemoglobin – Iron in nonreduced-state and not capable of binding oxygen – If greater than 10%, individuals appear cyanotic (blue color) – Can be induced by aniline drugs or hemoglobin M • Sulfhemoglobin – Affinity for oxygen is lower – Irreversible; green pigment – Exposure to sulfur chemicals, drugs
Hemolysis • Premature destruction of R B C’s • Extravascular 90% – RES system: spleen, liver, lymph nodes, bone marrow – Releases heme and globin contents to be recycled • Intravascular – Lysed directly into blood vessels
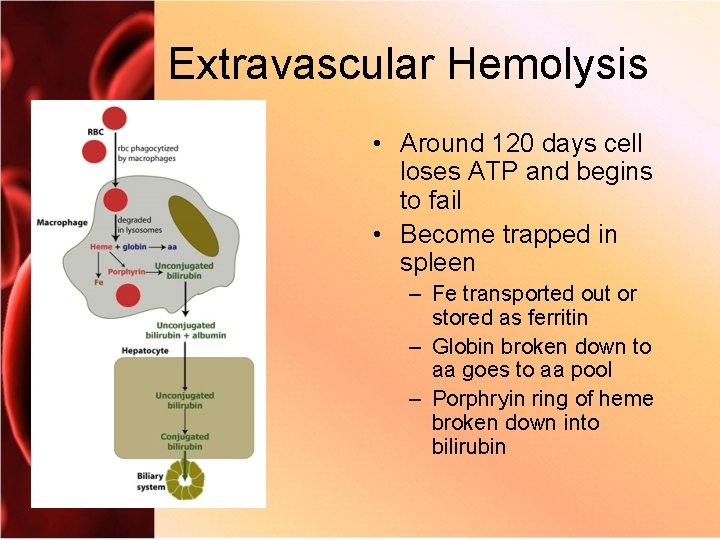
Extravascular Hemolysis • Around 120 days cell loses ATP and begins to fail • Become trapped in spleen – Fe transported out or stored as ferritin – Globin broken down to aa goes to aa pool – Porphryin ring of heme broken down into bilirubin
Laboratory Values Associated with Extravascular Hemolysis • • ↓ Hgb, Hct, RBC count ↑ Reticulocyte count Polychromasia in peripheral smear ↑ Serum bilirubin ↓ Haptoglobin ↑ LDH Spherocytes
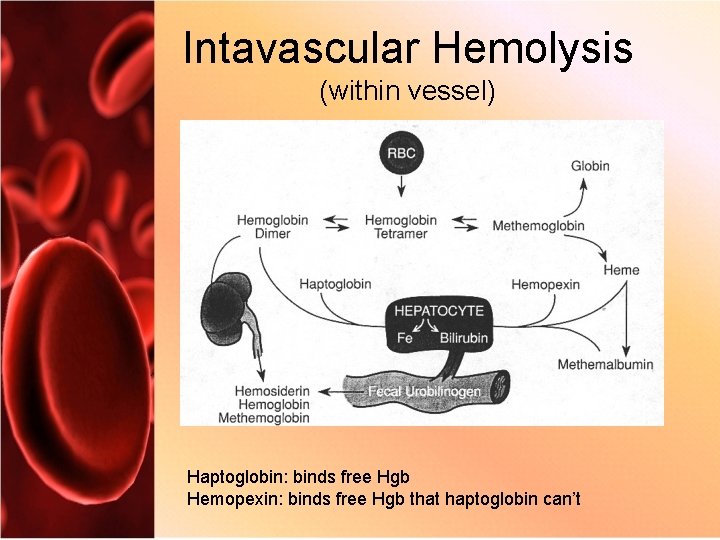
Intavascular Hemolysis (within vessel) Haptoglobin: binds free Hgb Hemopexin: binds free Hgb that haptoglobin can’t
Laboratory Values Associated with Intraavascular Hemolysis • • ↓ Hgb, Hct, R B C count ↑ Serum bilirubin ↓ Serum haptoglobin Hemoglobinemia Hemoglobinuria Possible ↑ reticulocytes ↑ LDH Schistocytes
- Slides: 18