Mixtures This powerpoints animations are best viewed in

Mixtures

This powerpoint’s animations are best viewed in full screen. Click on slideshow and play from start.

What is a mixture? A mixture is a _________ combination of two or more substances. Substances Sugar Flour Eggs Chocolate Substances Orange concentrate Sugar Water Substances Crust Cheese Sauce Mushrooms Pepperoni substance If there is only one substance, we call itpure a _________.

There are 2 types of mixtures to know: A heterogeneous mixture is made of two or more substances, where we can see the different substances just by looking at it. Salad Bowl of candy Oil and water Soft drink

There are 2 types of mixtures to know: A homogeneous mixture is made of two or more substances, but we can only see what appears to be one substance. Sugar and water Brass (copper and zinc) Air Steel (iron and carbon)

Particles in Mixtures In a heterogeneous mixture, the particles of each substance stay together. In a homogeneous mixture, the particles of each substance are spread out evenly.

More on homogeneous mixtures A homogeneous mixture always has 2 parts: • SOLVENT: What’s doing the dissolving (usually water) • SOLUTE: What’s being dissolved.

Let’s make a homogeneous mixture!
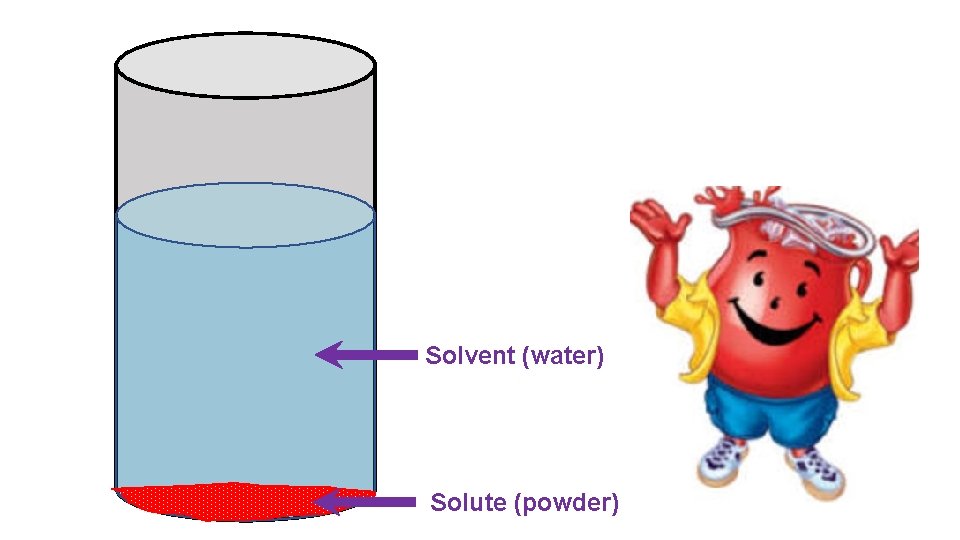
Solvent (water) Solute (powder)
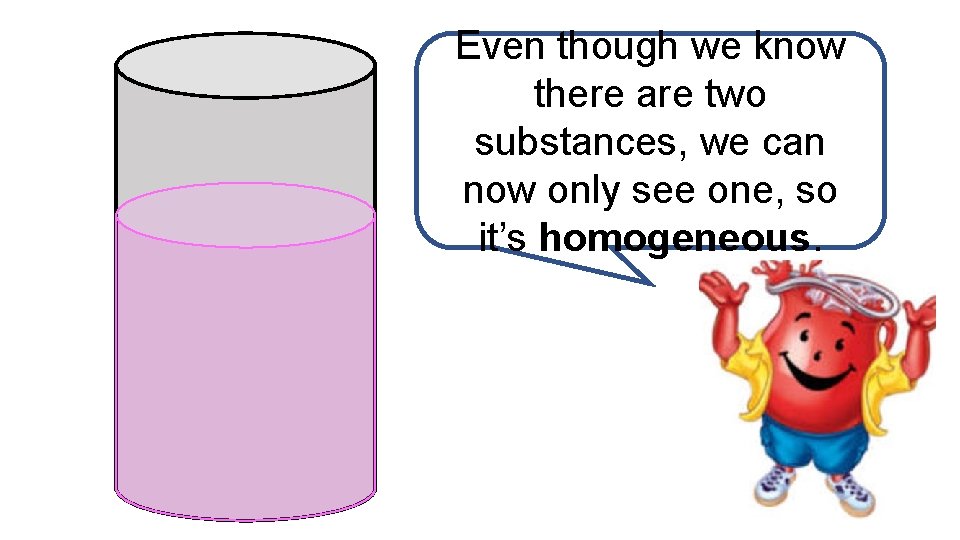
Even though we know there are two substances, we can now only see one, so it’s homogeneous.
Oh yeah!

! e True or False Once you make a mixture, it is impossible to separate its components. F s l a All mixtures can be separated!
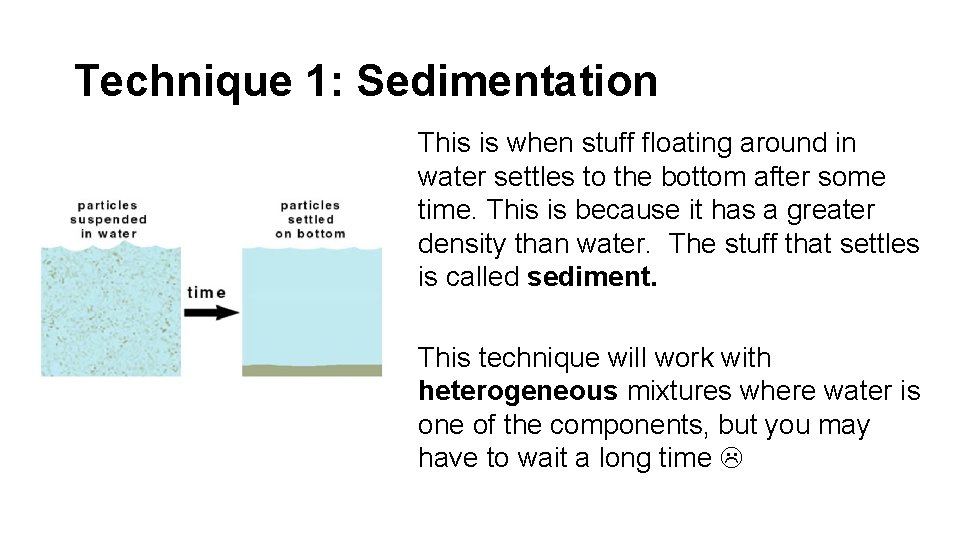
Technique 1: Sedimentation This is when stuff floating around in water settles to the bottom after some time. This is because it has a greater density than water. The stuff that settles is called sediment. This technique will work with heterogeneous mixtures where water is one of the components, but you may have to wait a long time
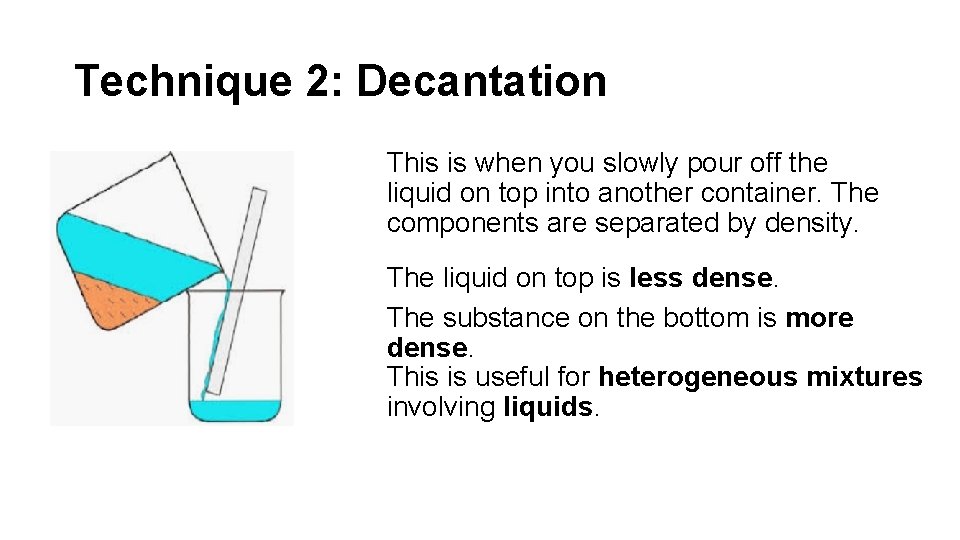
Technique 2: Decantation This is when you slowly pour off the liquid on top into another container. The components are separated by density. The liquid on top is less dense. The substance on the bottom is more dense. This is useful for heterogeneous mixtures involving liquids.
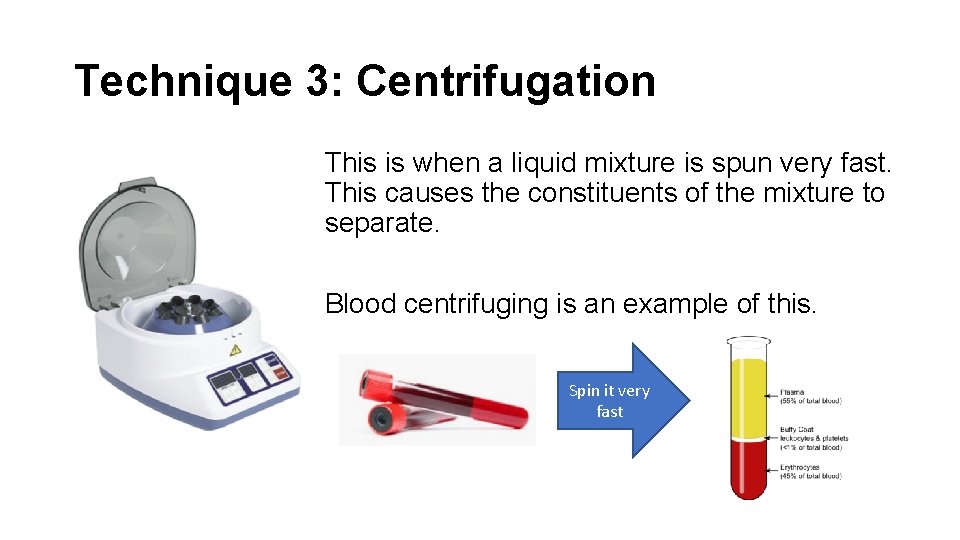
Technique 3: Centrifugation This is when a liquid mixture is spun very fast. This causes the constituents of the mixture to separate. Blood centrifuging is an example of this. Spin it very fast
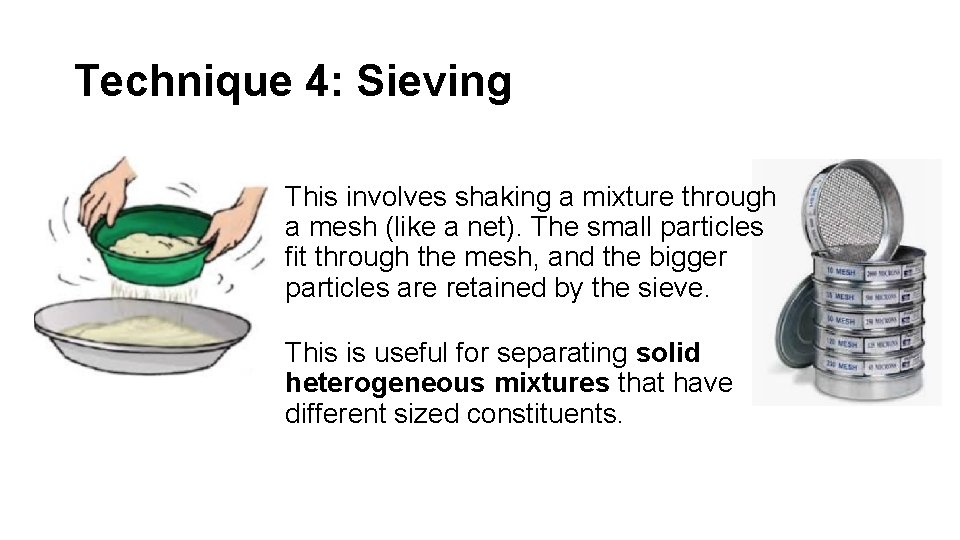
Technique 4: Sieving This involves shaking a mixture through a mesh (like a net). The small particles fit through the mesh, and the bigger particles are retained by the sieve. This is useful for separating solid heterogeneous mixtures that have different sized constituents.
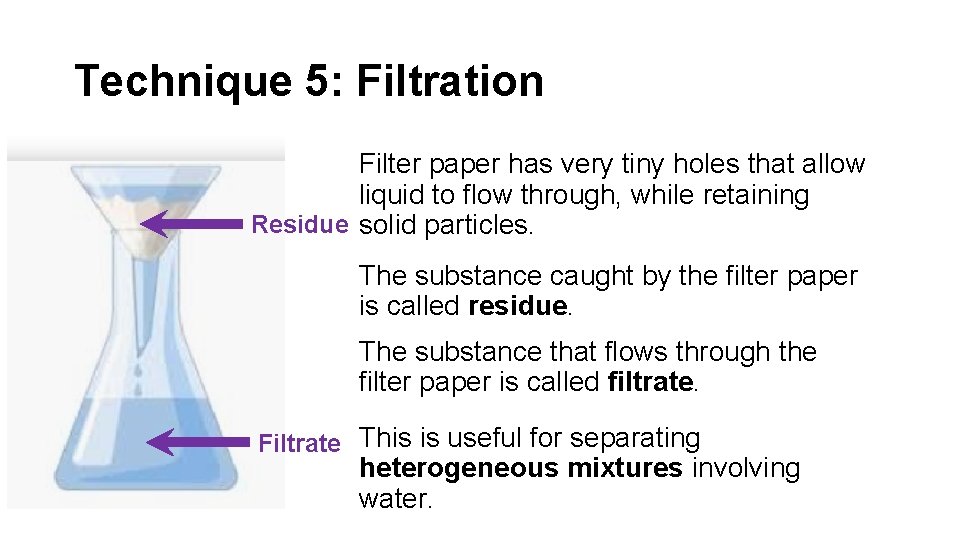
Technique 5: Filtration Filter paper has very tiny holes that allow liquid to flow through, while retaining Residue solid particles. The substance caught by the filter paper is called residue. The substance that flows through the filter paper is called filtrate. Filtrate This is useful for separating heterogeneous mixtures involving water.
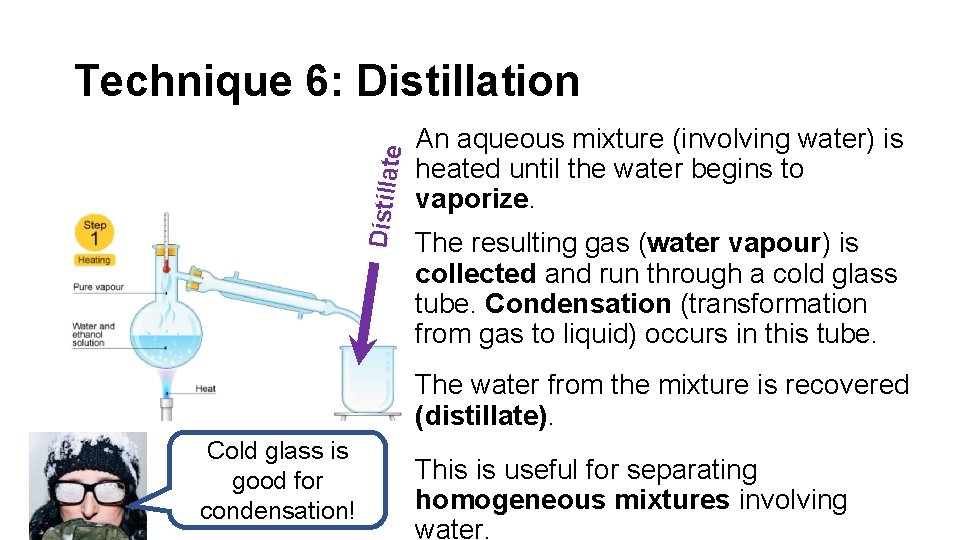
Distillat e Technique 6: Distillation An aqueous mixture (involving water) is heated until the water begins to vaporize. The resulting gas (water vapour) is collected and run through a cold glass tube. Condensation (transformation from gas to liquid) occurs in this tube. The water from the mixture is recovered (distillate). Cold glass is good for condensation! This is useful for separating homogeneous mixtures involving water.
- Slides: 18