Mixtures Solubility and Solutions A substance is matter

Mixtures, Solubility, and Solutions

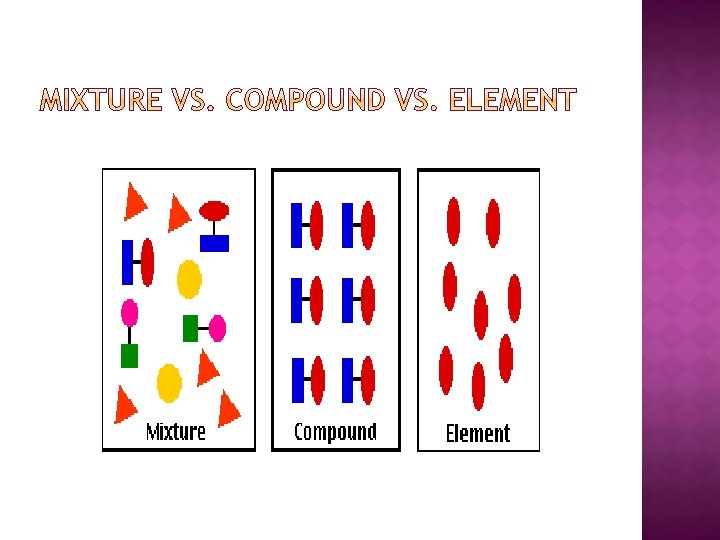
A substance is matter with composition that is always the same Two major types of substances Elements Compounds

Elements are substances that consist of just one type of atom Elements are found on the periodic table Each element has its own characteristic properties. These include boiling/melting point, density, reactivity, flammability, etc. An element may share one or two properties with another element, but it will not share all its properties with another element. There will be specific properties for each element.
Compounds are substances containing atoms of two or more different elements chemically bonded together Occurs as a result of a chemical reaction Compounds are represented by chemical formulas Ex. Na. Cl, CO 2, H 2 O
Properties of compounds Compounds have a SET RATIO Ex. H 2 O (water) vs H 2 O 2 (hydrogen peroxide) Each compound has its own physical properties and chemical properties. The properties of a compound are different from the properties of the elements that form it. Sodium Explosive soft metal + Chlorine Yellow Piousness Gas Table Salt
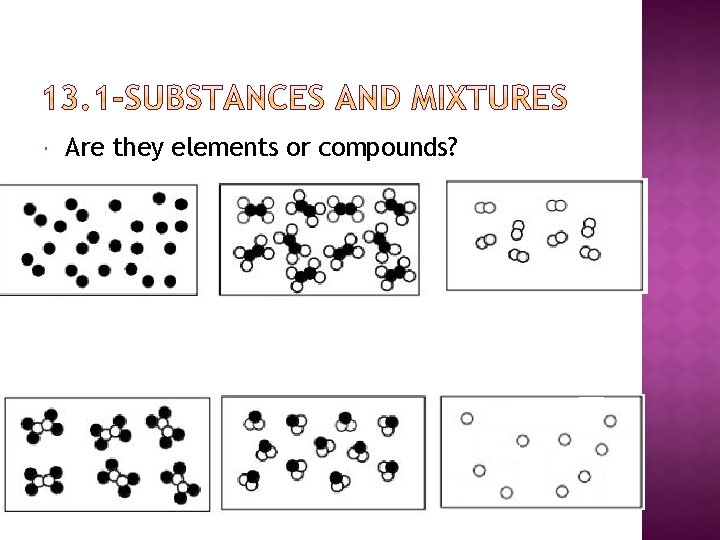
Are they elements or compounds?

What is a mixture? A Mixture is two or more substances that are physically blended but are not chemically bonded together No chemical reaction No compound formed Each substance keeps its original identity No fixed ratio—NOT always the same
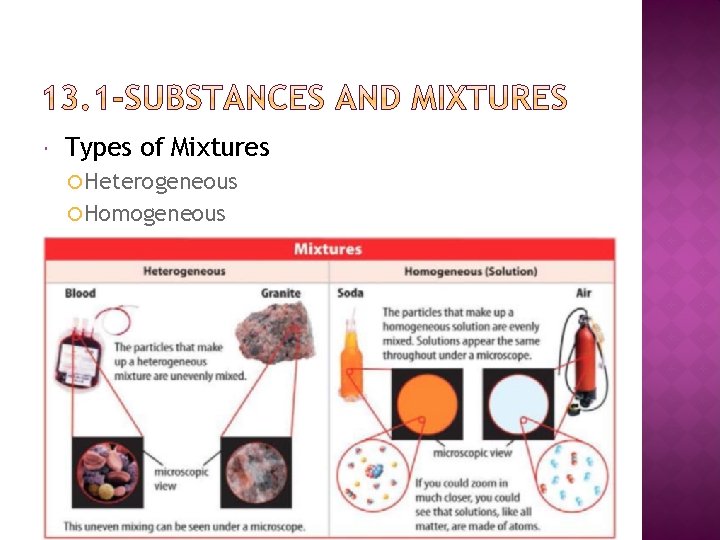
Types of Mixtures Heterogeneous Homogeneous
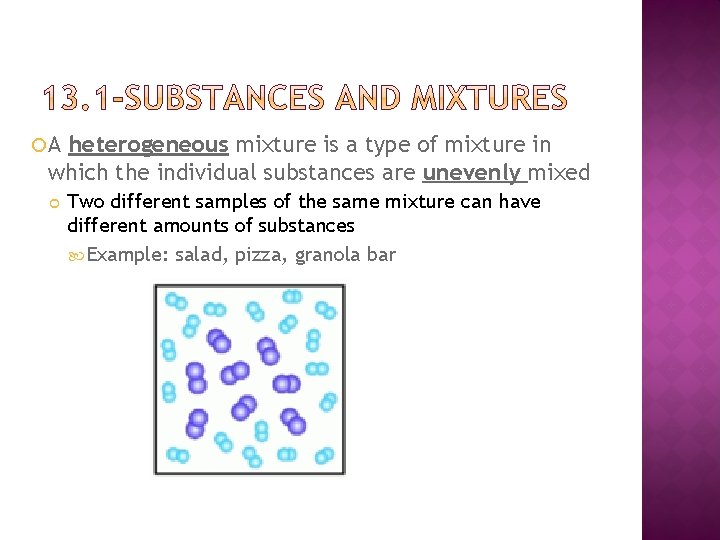
A heterogeneous mixture is a type of mixture in which the individual substances are unevenly mixed Two different samples of the same mixture can have different amounts of substances Example: salad, pizza, granola bar

Types of heterogeneous mixtures Suspension A suspension is a mixture in which particles are large enough to be dispersed, but they settle out over time Can be filtered, can scatter light Colloid A colloid is a mixture in which the particles are medium in size and dispersed throughout but are not heavy enough to settle out Cannot be filtered, can scatter light
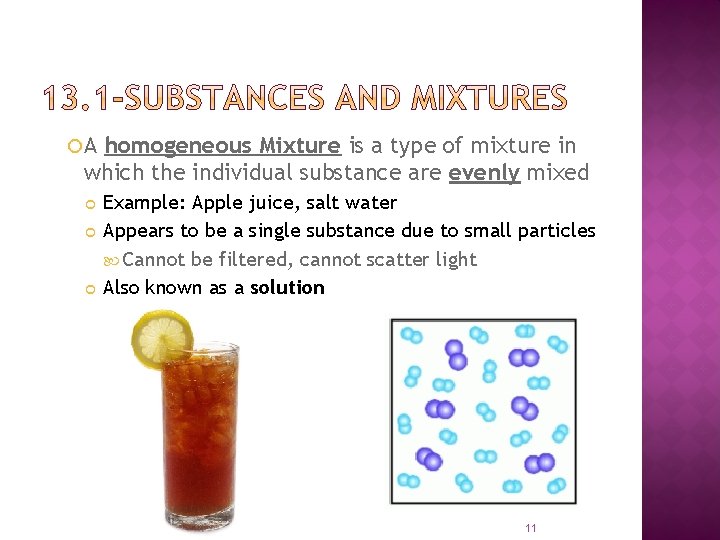
A homogeneous Mixture is a type of mixture in which the individual substance are evenly mixed Example: Apple juice, salt water Appears to be a single substance due to small particles Cannot be filtered, cannot scatter light Also known as a solution 11
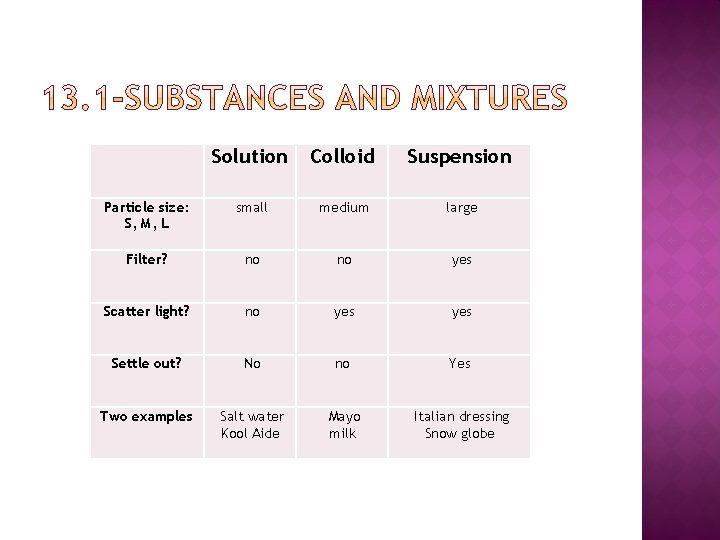
Solution Colloid Suspension Particle size: S, M, L small medium large Filter? no no yes Scatter light? no yes Settle out? No no Yes Two examples Salt water Kool Aide Mayo milk Italian dressing Snow globe
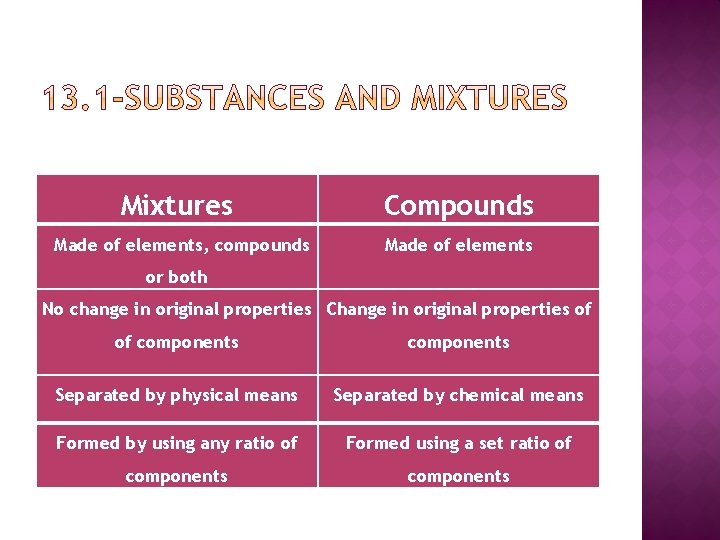
Mixtures Compounds Made of elements, compounds Made of elements or both No change in original properties Change in original properties of of components Separated by physical means Separated by chemical means Formed by using any ratio of Formed using a set ratio of components
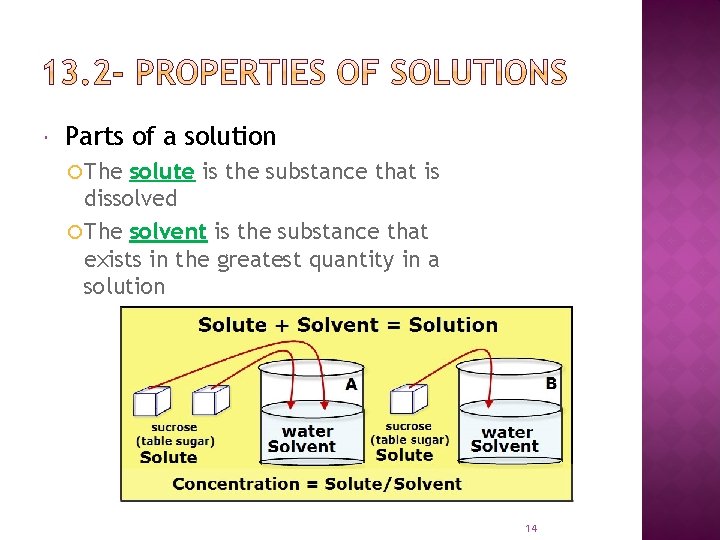
Parts of a solution The solute is the substance that is dissolved The solvent is the substance that exists in the greatest quantity in a solution 14

� Concentration of Solutions ◦ The concentration is the amount of a particular solute in a given amount of solvent �Units: g/m. L ◦ Lots of solute concentrated ◦ Little solute dilute
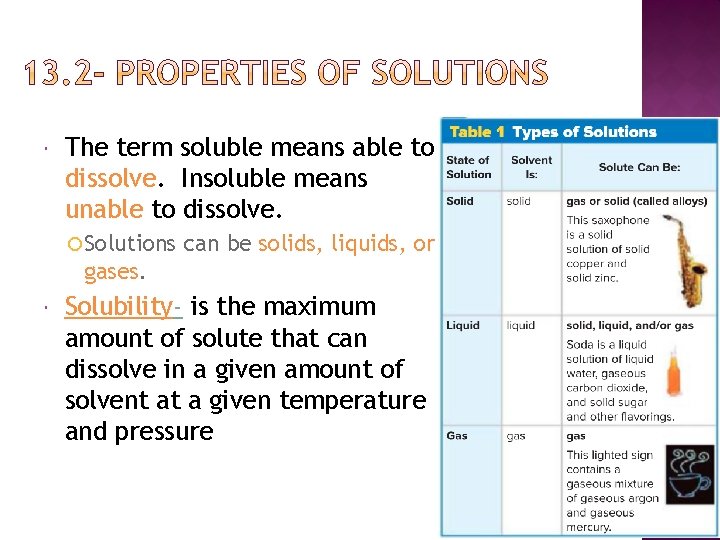
The term soluble means able to dissolve. Insoluble means unable to dissolve. Solutions can be solids, liquids, or gases. Solubility- is the maximum amount of solute that can dissolve in a given amount of solvent at a given temperature and pressure

Solubility Saturated solutions are solutions that contain the maximum amount of solute that the solution can hold at a given temperature and pressure Unsaturated solutions are solutions that can still dissolve more solute at a given temperature and pressure

How does temperature affect solubility? For liquid solvents A higher temperature decreases the solubility of a gas A higher temperature inceases the solubility of a solid or liquid How does pressure affect solubility? A higher pressure increases the solubility of a gas
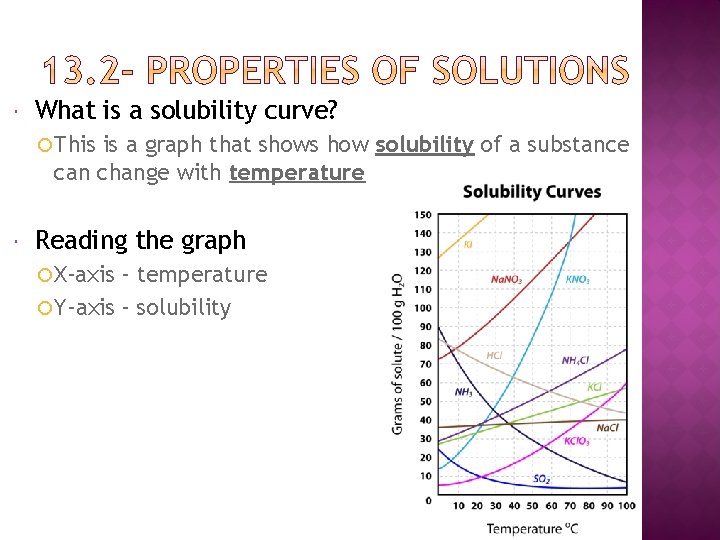
What is a solubility curve? This is a graph that shows how solubility of a substance can change with temperature Reading the graph X-axis – temperature Y-axis – solubility
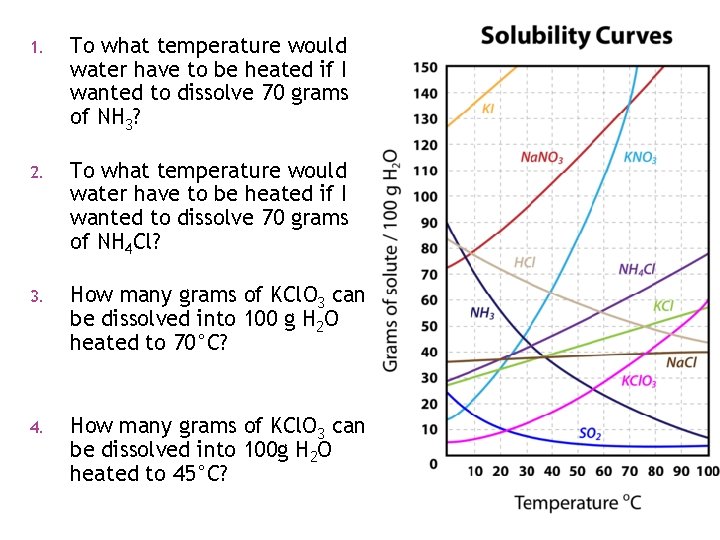
1. To what temperature would water have to be heated if I wanted to dissolve 70 grams of NH 3? 2. To what temperature would water have to be heated if I wanted to dissolve 70 grams of NH 4 Cl? 3. How many grams of KCl. O 3 can be dissolved into 100 g H 2 O heated to 70°C? 4. How many grams of KCl. O 3 can be dissolved into 100 g H 2 O heated to 45°C?

How fast a solute dissolves All speed up dissolving
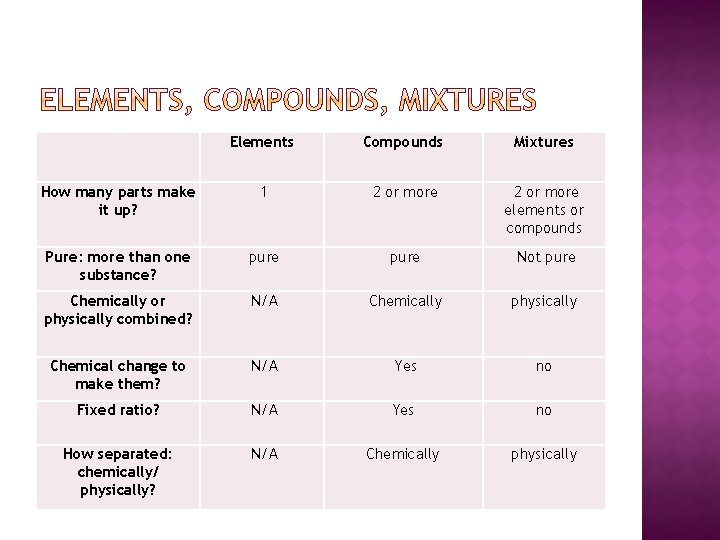
Elements Compounds Mixtures How many parts make it up? 1 2 or more elements or compounds Pure: more than one substance? pure Not pure Chemically or physically combined? N/A Chemically physically Chemical change to make them? N/A Yes no Fixed ratio? N/A Yes no How separated: chemically/ physically? N/A Chemically physically
- Slides: 23