Mixtures of Matter I Mixtures A A combination

Mixtures of Matter

I. Mixtures A. A combination of two or more pure substances B. Each pure substance retains its individual chemical properties

Question Time • What are some examples of mixtures? • What is the difference between Chicken noodle soup mixture and vinegar mixture? • What science prefix would we use for each? 3

II. Types of Mixtures A. Heterogeneous (hetero = different) 1. Does not have uniform composition 2. individual substances remain distinct B. Homogenous (homo = same) 1. Has uniform composition throughout 2. always has a single phase 3. Also called a solution

Question time • Can we have mixtures of metal? • What are some examples? What do we call these metals? 5

III. Solid-solid solution (Alloy) A. A homogeneous mixture B. A type of solid- solid solution is called an alloy C. Brass is an example of an alloy made of zinc and copper + = Brass

Question Time • What is a mixture? • What are the two types of mixtures • What is the difference between a homogenous and a heterogeneous mixture? • What is a solid-solid homogeneous mixture called?

Question Time State whether it is homogeneous or heterogeneous mixture • Orange juice with pulp • Raisin muffin • Steel • Salt water solution • Paper towel • Mustard

Question Time • What do we do to water to get impurities out? 9
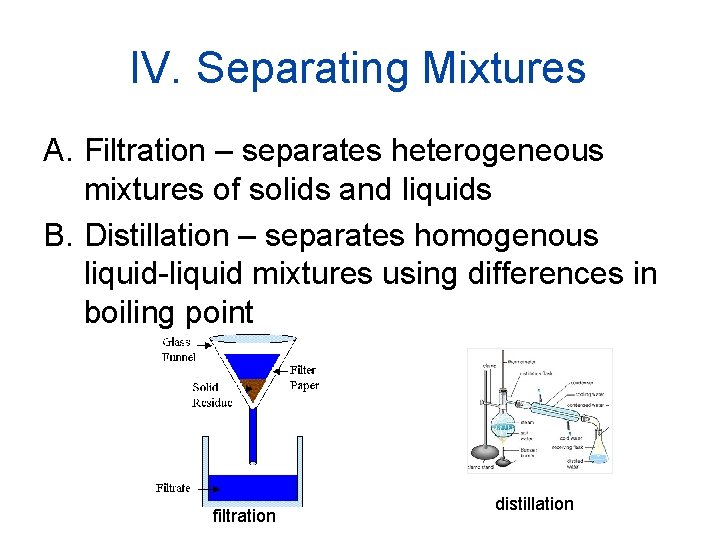
IV. Separating Mixtures A. Filtration – separates heterogeneous mixtures of solids and liquids B. Distillation – separates homogenous liquid-liquid mixtures using differences in boiling point filtration distillation
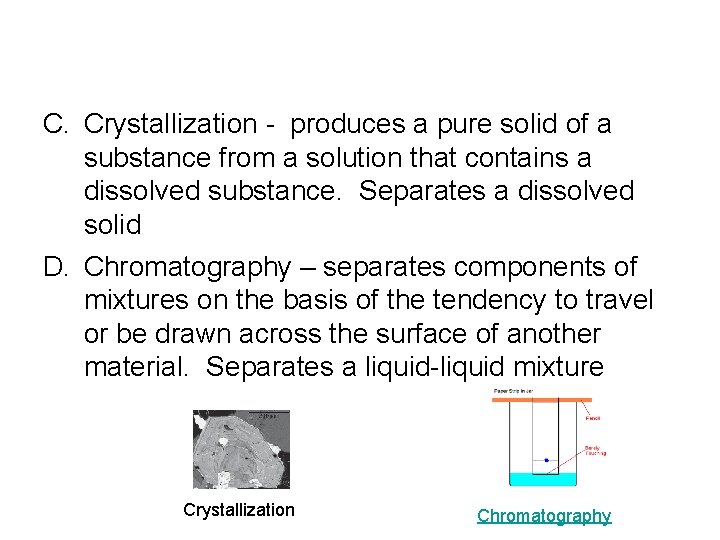
C. Crystallization - produces a pure solid of a substance from a solution that contains a dissolved substance. Separates a dissolved solid D. Chromatography – separates components of mixtures on the basis of the tendency to travel or be drawn across the surface of another material. Separates a liquid-liquid mixture Crystallization Chromatography

Question Time • How would you separate sand from water? • How you separate alcohol from water? (They have different boiling points) • How would you separate sugar dissolved in water? • How would you separate a mixture of ink?
- Slides: 12