Mixtures of Gases Most Important Concept Gases act





- Slides: 10

Mixtures of Gases

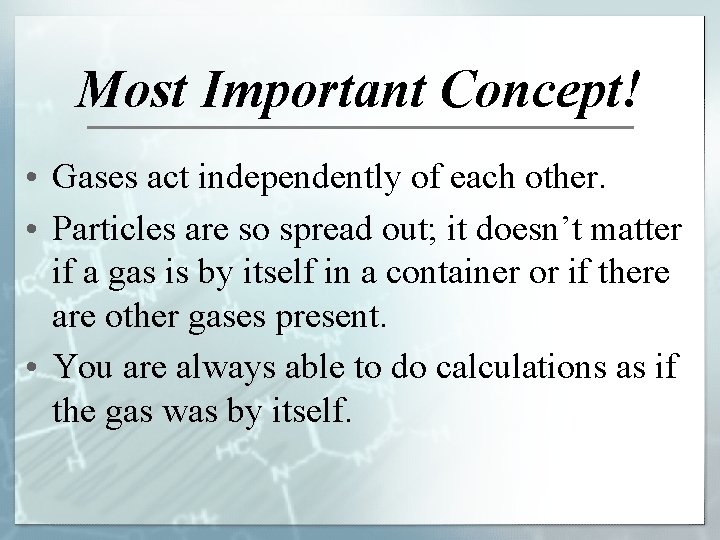
Most Important Concept! • Gases act independently of each other. • Particles are so spread out; it doesn’t matter if a gas is by itself in a container or if there are other gases present. • You are always able to do calculations as if the gas was by itself.

Partial Pressure What is partial pressure? IT’S PRESSURE! So, why is it called “partial” pressure? Because, in a mixture of gases, the pressure of one gas is only PART of the total pressure. Does that change my calculations? No and Yes!

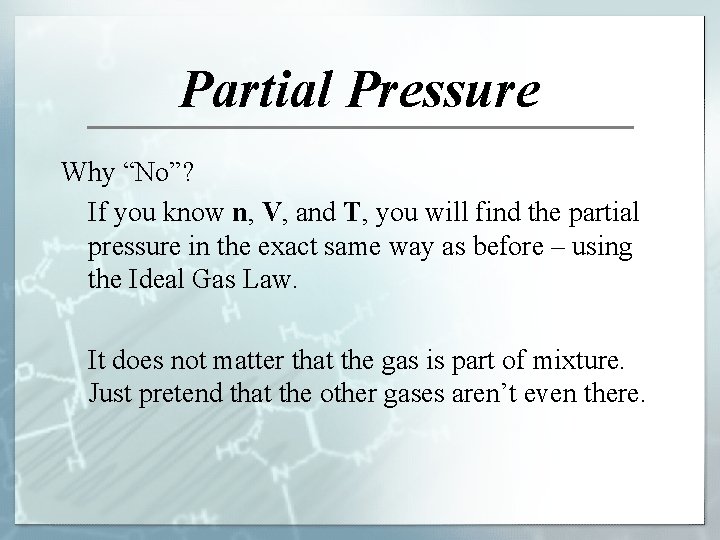
Partial Pressure Why “No”? If you know n, V, and T, you will find the partial pressure in the exact same way as before – using the Ideal Gas Law. It does not matter that the gas is part of mixture. Just pretend that the other gases aren’t even there.

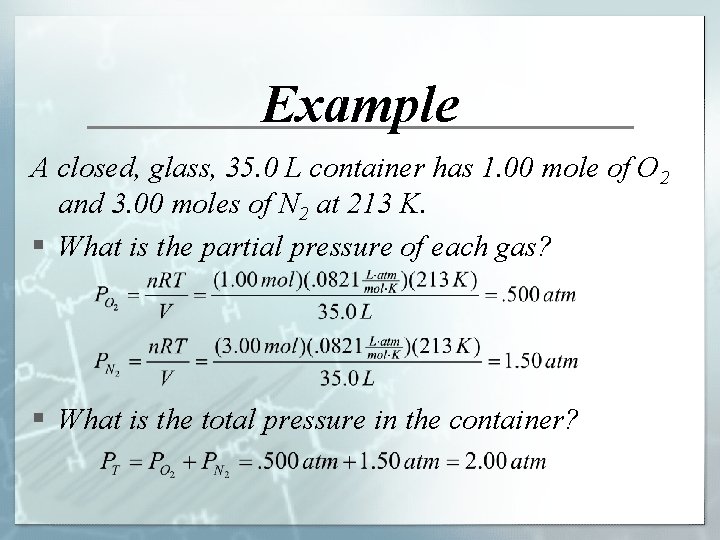
Example A closed, glass, 35. 0 L container has 1. 00 mole of O 2 and 3. 00 moles of N 2 at 213 K. § What is the partial pressure of each gas? § What is the total pressure in the container?

Partial Pressure Why “Yes”? If a gas is part of a mixture, it allows us to find its partial pressure in other ways, even if we don’t know the temperature or the volume.

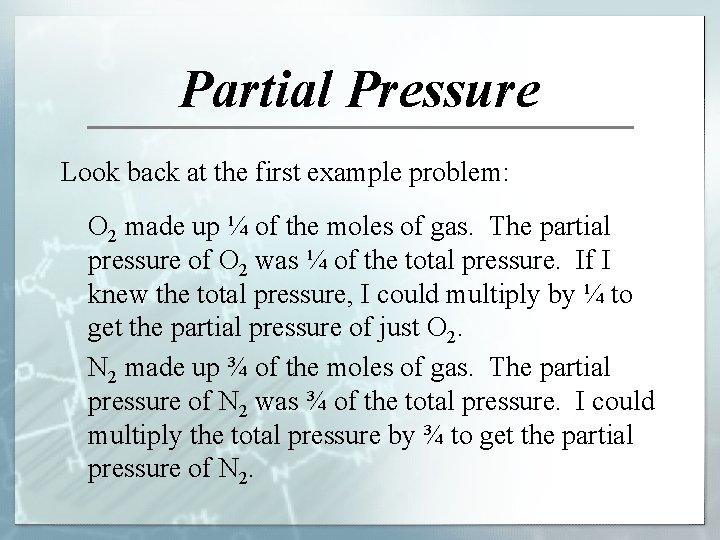
Partial Pressure Look back at the first example problem: O 2 made up ¼ of the moles of gas. The partial pressure of O 2 was ¼ of the total pressure. If I knew the total pressure, I could multiply by ¼ to get the partial pressure of just O 2. N 2 made up ¾ of the moles of gas. The partial pressure of N 2 was ¾ of the total pressure. I could multiply the total pressure by ¾ to get the partial pressure of N 2.

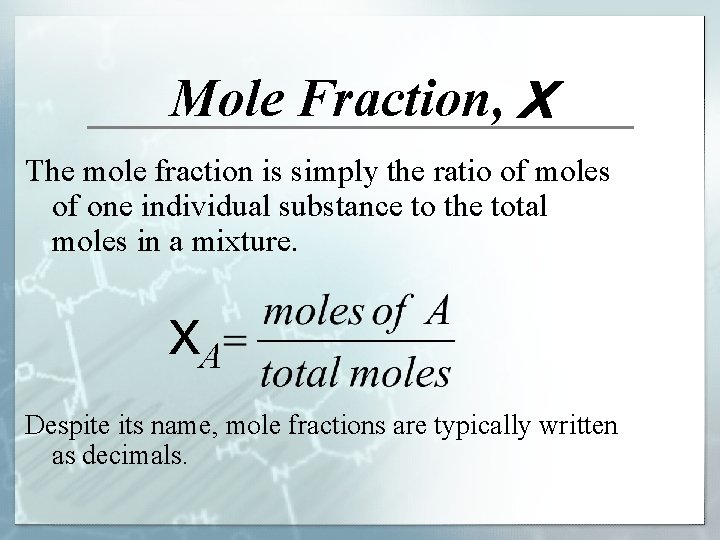
Mole Fraction, X The mole fraction is simply the ratio of moles of one individual substance to the total moles in a mixture. XA Despite its name, mole fractions are typically written as decimals.

Mole Fraction, X As shown from our example, the mole fraction has the same value as the ratio of partial pressure to total pressure. We can use this to find partial pressures even if we don’t know the temperature and/or volume of our container. P A = X AP T

Example A container with a total pressure of 600. mm. Hg contains 2. 00 moles of He, 1. 50 moles of Ne, and 0. 50 moles of Ar. Find the partial pressure of each gas. P A = X AP T