Mixtures Matter Substances Elements Compounds Mixtures Heterogeneous Mixtures

Mixtures
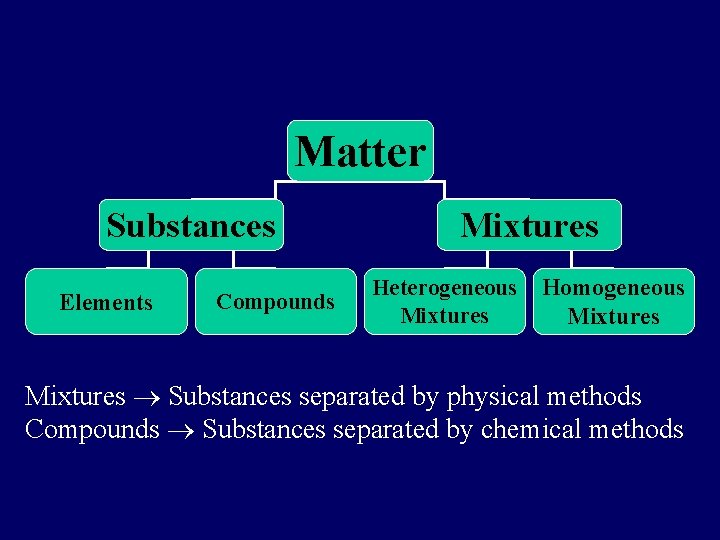
Matter Substances Elements Compounds Mixtures Heterogeneous Mixtures Homogeneous Mixtures Substances separated by physical methods Compounds Substances separated by chemical methods

Mixtures • Combo of 2 or more pure substances • Physically combined NOT chemically combined • Each substance retains its own identity and properties

Mixtures • Variable composition • No unique properties • Ex: sugar and sand mixed together • Separated by physical methods • May be homogeneous or heterogeneous

Types of Mixtures • Heterogeneous: See boundary or regions that look different Ex: • colloids • suspensions • ice water • granite • cereals

Heterogeneous mixtures: cereal/granite Colloid: milk Suspension: smoke/smog Heterogeneous mixture: ice water

• Homogeneous: constant composition throughout, single phase Ex: • Solutions (all 3 phases) • Air • Windex • kool-aid

Homogeneous Solutions: Drink mix Air Window cleaner

Hints for Mixtures • Solutions (gas & liquid phases) transmit light • No particles big enough to scatter light • Look translucent (see-through) • Suspensions look cloudy – scatter light • Particles big enough to scatter light • Settle upon standing

Where does this liquid fit? Homogeneous? Heterogeneous?
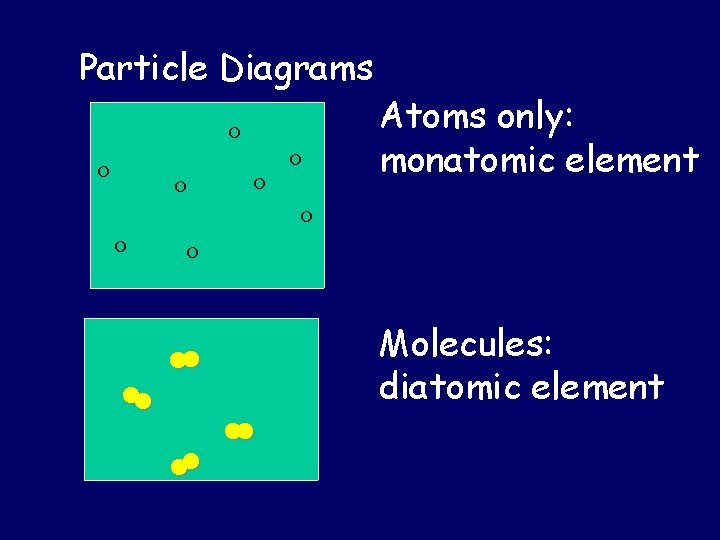
Particle Diagrams Atoms only: monatomic element Molecules: diatomic element
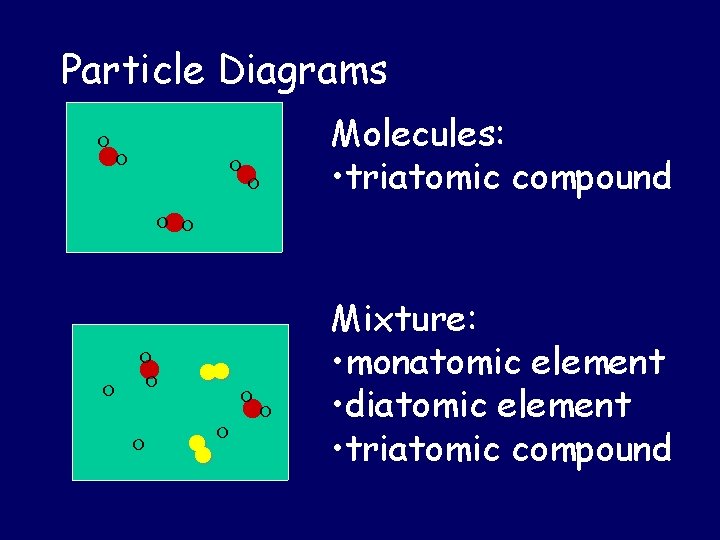
Particle Diagrams Molecules: • triatomic compound Mixture: • monatomic element • diatomic element • triatomic compound

Conservation of Mass • Mass before = Mass after • # atoms before = # atoms after

Separating Mixtures • Physically combined • Separation based on physical properties – Sorting: Appearance – Filtration: Size – Distillation: Boiling Point – Crystallization: Solubility – Magnet: Magnetization – Chromatography: “Travel” ability
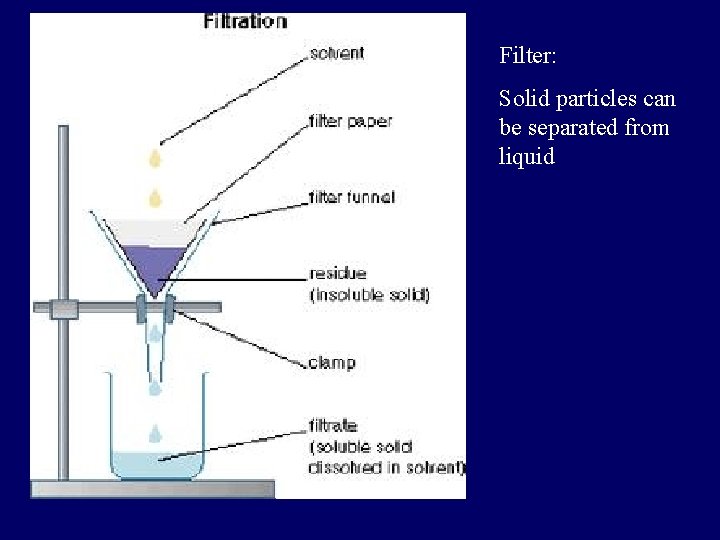
Filter: Solid particles can be separated from liquid
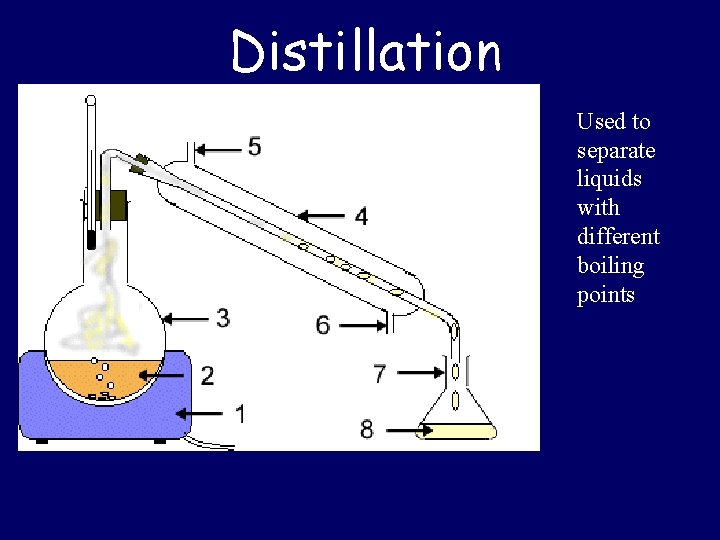
Distillation Used to separate liquids with different boiling points
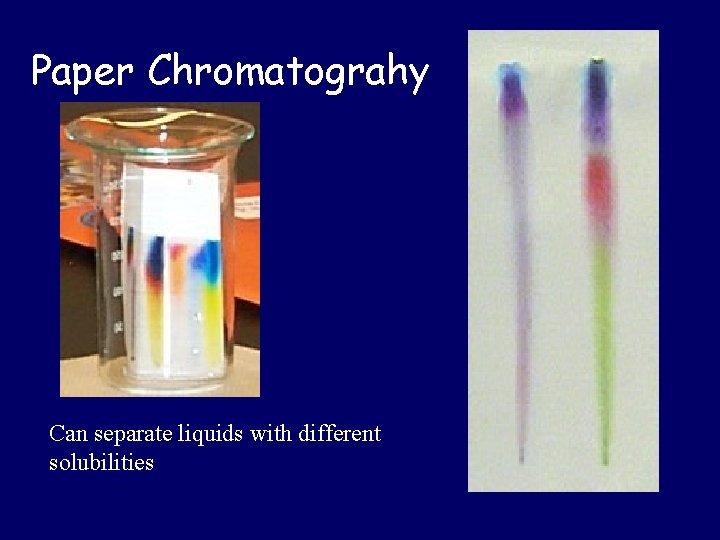
Paper Chromatograhy Can separate liquids with different solubilities

Crystallization Can separate solids dissolved in liquids
- Slides: 18