MIXTURES Ch 5 3 PROPERTIES OF MIXTURES A












- Slides: 16

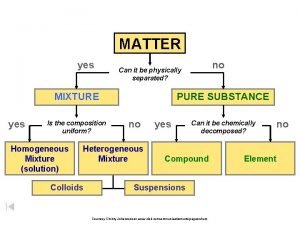
MIXTURES Ch. 5 - 3

PROPERTIES OF MIXTURES A mixture is a group of two or more substances that are not chemically combined. • Example: Toppings on a pizza, glass of tea, bag of assorted cookies, & air

NO CHEMICAL CHANGES Each substance in the mixture has the same properties it had before. • Mixtures can be physically separated.

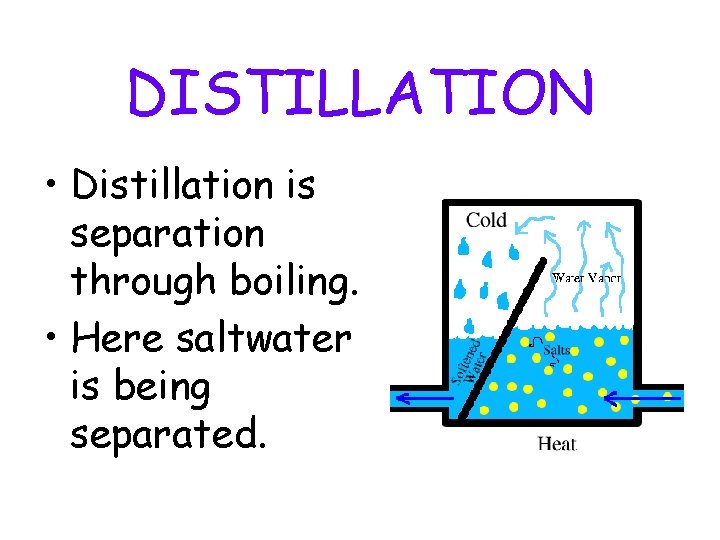
DISTILLATION • Distillation is separation through boiling. • Here saltwater is being separated.

MAGNETS • Magnets can be used to separate a mixture of metals. • Some metals, like aluminum, aren’t magnetic.

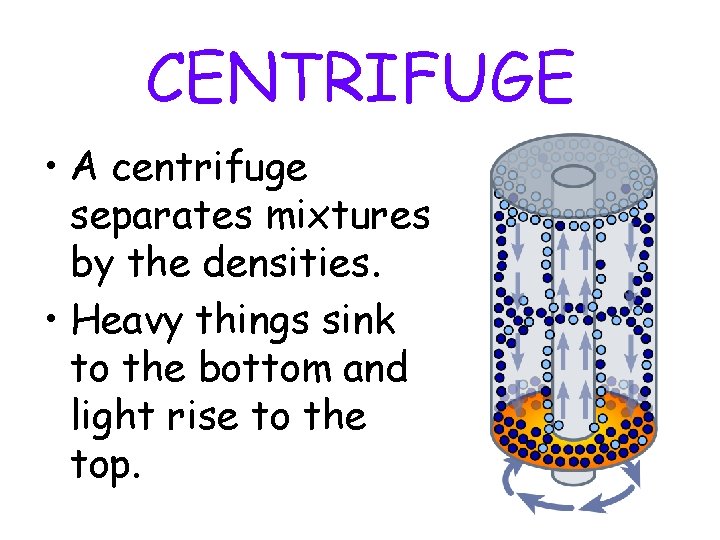
CENTRIFUGE • A centrifuge separates mixtures by the densities. • Heavy things sink to the bottom and light rise to the top.

Ratio of the Components The substances in a mixture do not have a definite ratio.

Heterogeneous Mixture • The different substances are easy to see. • One doesn’t dissolve into the other.

Homogeneous Mixture • It has the same appearance throughout the mixture. • The tea has dissolved into the water.

SOLUTIONS • A solution is a mixture that appears to be a single substance but is made of two or more substances that are distributed evenly.

Particles in Solutions • The particles in a solution are so small they will not come out of the solution. • They can not be filtered out. • They will not scatter light.

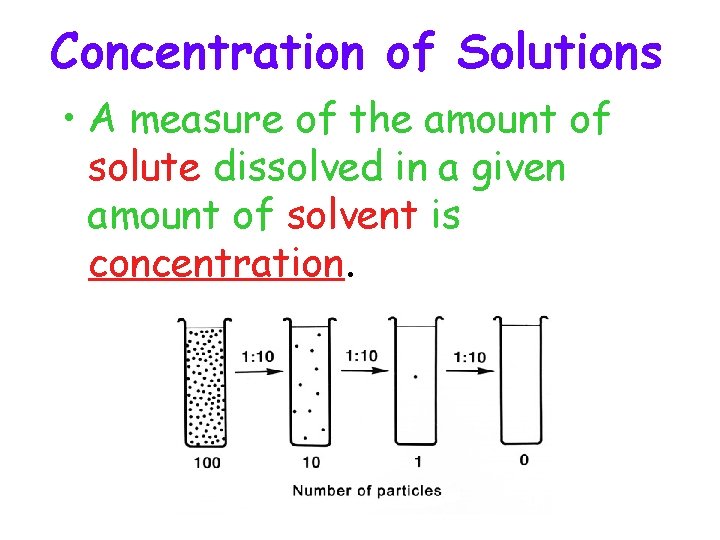
Concentration of Solutions • A measure of the amount of solute dissolved in a given amount of solvent is concentration.

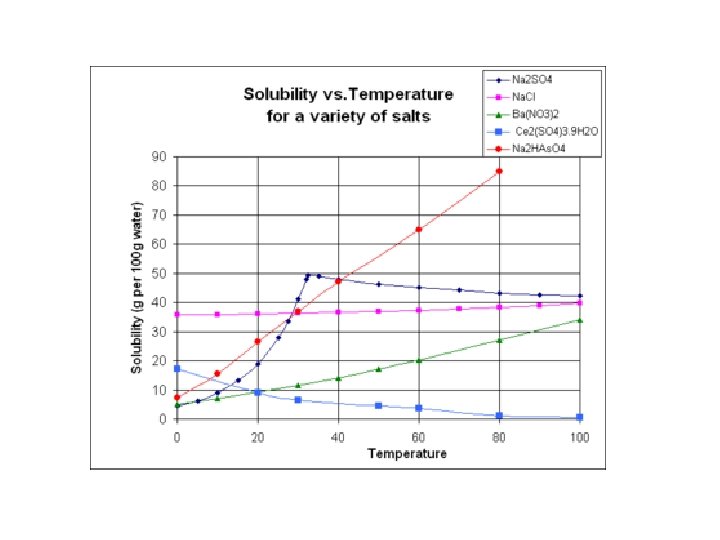
Solubility * Solubility is the ability of one substance to dissolve into another. • It increases as temperature increases. • Like sugar into water

Examples of Solutions Soft drinks are liquid solutions. Air is a solution that is a gas. Steel is a solid solution.


LET’S TRY IT!
 Properties of mixtures
Properties of mixtures Extensive examples
Extensive examples Is smell a physical property
Is smell a physical property Examples of flour mixtures
Examples of flour mixtures Can sulfur and water be separated by filtration
Can sulfur and water be separated by filtration Common homogeneous mixtures
Common homogeneous mixtures Mixtures and solutions quiz
Mixtures and solutions quiz Are solutions homogeneous
Are solutions homogeneous Are all solutions homogeneous mixtures
Are all solutions homogeneous mixtures Elements compounds and mixtures quiz
Elements compounds and mixtures quiz Variable composition
Variable composition Different methods of separating mixtures
Different methods of separating mixtures Alligation rule for 2 mixtures
Alligation rule for 2 mixtures Separation funnel diagram
Separation funnel diagram Separating mixtures grade 7
Separating mixtures grade 7 Mixtures
Mixtures 14.4 gases mixtures and movements answer key
14.4 gases mixtures and movements answer key