Mixing of gases 1 Gibbs Paradox If N

Mixing of gases 1: Gibbs’ Paradox If N molecules of an ideal monatomic gas make a free expansion from a V to 2 V, ΔU = 0, so that ΔS = Sf – Si = Nk ln(2 V/V) = Nk ln 2. If the container of volume 2 V is now redivided into equal parts, so that (N/2) molecules are in each half, then ΔS’ = Sf’ – Si’ = 2(N/2)k ln(V/2 V) = – Nk ln 2. This is Gibbs’ paradox, which is removed by assuming that the molecules are indistiguishable; i. e. that ω(U, V, N) = B(N) U 3 N/2 VN/N!. The term Nk ln. V in the expression for S is replaced by Nk ln(V/N!), where (by Stirling’s theorem), 1 Nk ln(V/N!) ≈ Nk [ln(V/N) + 1].
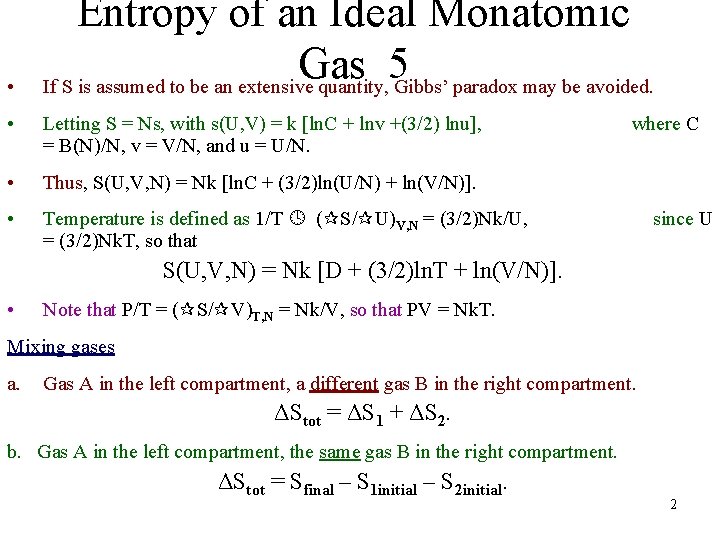
• Entropy of an Ideal Monatomic Gas 5 If S is assumed to be an extensive quantity, Gibbs’ paradox may be avoided. • Letting S = Ns, with s(U, V) = k [ln. C + lnv +(3/2) lnu], = B(N)/N, v = V/N, and u = U/N. • Thus, S(U, V, N) = Nk [ln. C + (3/2)ln(U/N) + ln(V/N)]. • Temperature is defined as 1/T ( S/ U)V, N = (3/2)Nk/U, = (3/2)Nk. T, so that where C since U S(U, V, N) = Nk [D + (3/2)ln. T + ln(V/N)]. • Note that P/T = ( S/ V)T, N = Nk/V, so that PV = Nk. T. Mixing gases a. Gas A in the left compartment, a different gas B in the right compartment. ΔStot = ΔS 1 + ΔS 2. b. Gas A in the left compartment, the same gas B in the right compartment. ΔStot = Sfinal – S 1 initial – S 2 initial. 2
- Slides: 2