Mixing and Solution When two different liquids are

- Slides: 13

Mixing and Solution Ø When two different liquids are mixed or when a gas or solid is dissolved into a liquid, bonds are broken between neighboring molecules. Ø Net release in energy will result when the bonds are broken and solution is form. Ø Suppose we mix 1 mol of pure liquid of sulfuric acid with water at specified temperature and pressure v The energy balance for this constant pressure-process is given by: where ΔH-the difference between the enthalpy of the solution at the specified temperature and pressure and the total enthalpy of the pure solute and solvent at the same T and P is the Heat of Solution at that temperature and pressure.

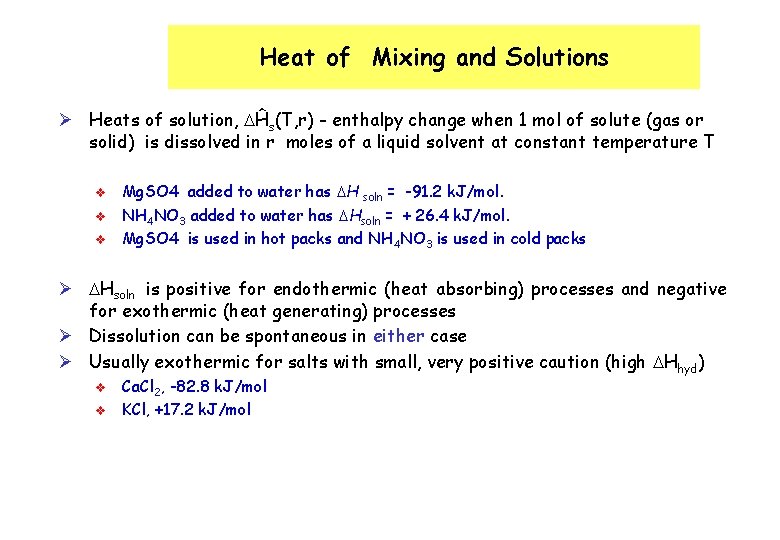
Heat of Mixing and Solutions Ø Heats of solution, Ĥs(T, r) - enthalpy change when 1 mol of solute (gas or solid) is dissolved in r moles of a liquid solvent at constant temperature T v v v Mg. SO 4 added to water has H soln = -91. 2 k. J/mol. NH 4 NO 3 added to water has Hsoln = + 26. 4 k. J/mol. Mg. SO 4 is used in hot packs and NH 4 NO 3 is used in cold packs Ø Hsoln is positive for endothermic (heat absorbing) processes and negative for exothermic (heat generating) processes Ø Dissolution can be spontaneous in either case Ø Usually exothermic for salts with small, very positive caution (high Hhyd) v v Ca. Cl 2, -82. 8 k. J/mol KCl, +17. 2 k. J/mol

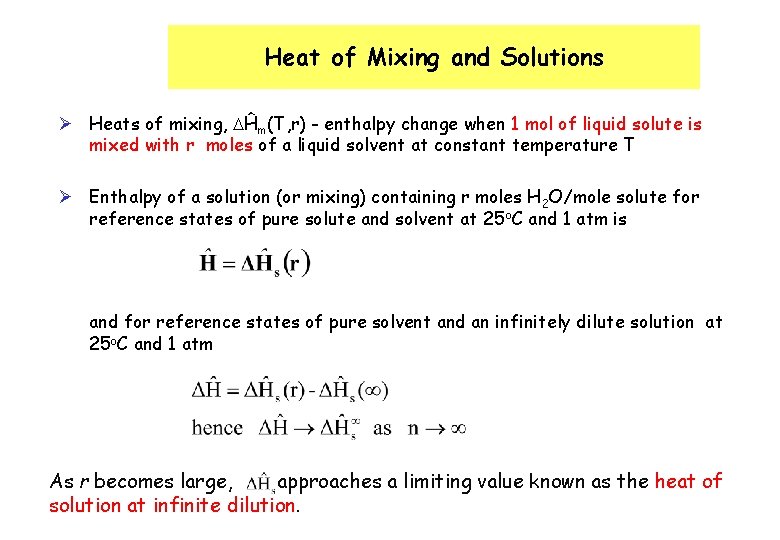
Heat of Mixing and Solutions Ø Heats of mixing, Ĥm(T, r) - enthalpy change when 1 mol of liquid solute is mixed with r moles of a liquid solvent at constant temperature T Ø Enthalpy of a solution (or mixing) containing r moles H 2 O/mole solute for reference states of pure solute and solvent at 25 o. C and 1 atm is and for reference states of pure solvent and an infinitely dilute solution at 25 o. C and 1 atm As r becomes large, approaches a limiting value known as the heat of solution at infinite dilution.

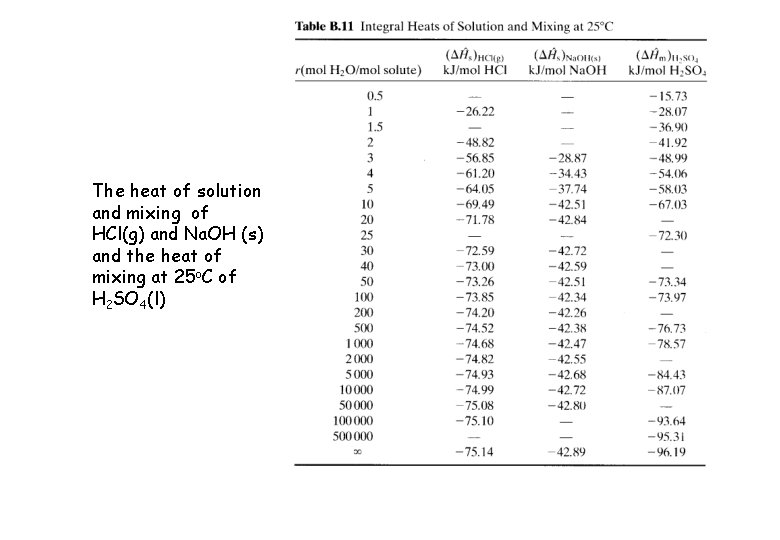
Heat of Mixing and Solutions Ø Heats of solution or mixing for an ideal mixture / solution (e. g. gas mixtures or liquid mixtures of structurally similar compounds, e. g. Paraffins, aromatics) is usually negligible, the enthalpy of mixtures is approximately Ø Aqueous solution of strong acids or bases of certain gases (HCl) or solids (Na. OH) heats of solution should be included in energy balance calculations Ø Data of the heats of solution is given in Perry’s Chemical Engineering Handbook on pp 2 -201 - 2 -204. Ø Some values of the heat of solution at 25 o. C of HCl(g) and Na. OH (s) and the heat of mixing at 25 o. C of H 2 SO 4(l) are given in Table B. 11, p. 653.

The heat of solution and mixing of HCl(g) and Na. OH (s) and the heat of mixing at 25 o. C of H 2 SO 4(l)

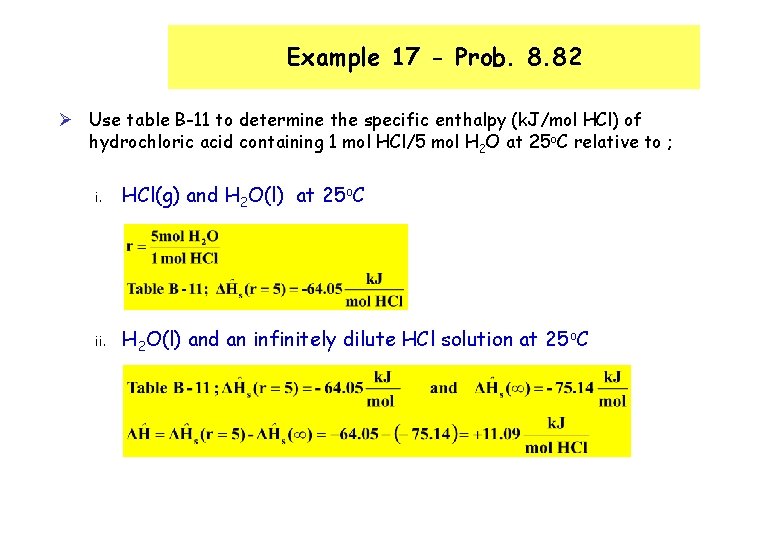
Example 17 - Prob. 8. 82 Ø Use table B-11 to determine the specific enthalpy (k. J/mol HCl) of hydrochloric acid containing 1 mol HCl/5 mol H 2 O at 25 o. C relative to ; i. HCl(g) and H 2 O(l) at 25 o. C ii. H 2 O(l) and an infinitely dilute HCl solution at 25 o. C

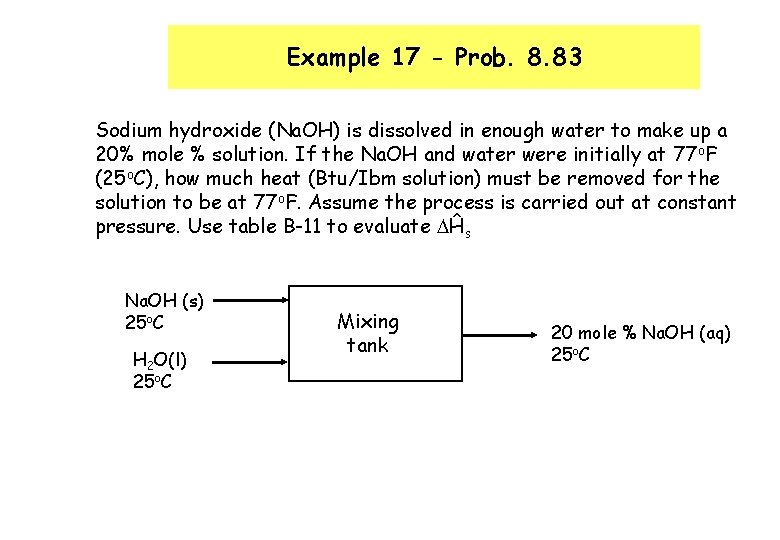
Example 17 - Prob. 8. 83 Sodium hydroxide (Na. OH) is dissolved in enough water to make up a 20% mole % solution. If the Na. OH and water were initially at 77 o. F (25 o. C), how much heat (Btu/Ibm solution) must be removed for the solution to be at 77 o. F. Assume the process is carried out at constant pressure. Use table B-11 to evaluate Ĥs Na. OH (s) 25 o. C H 2 O(l) 25 o. C Mixing tank 20 mole % Na. OH (aq) 25 o. C

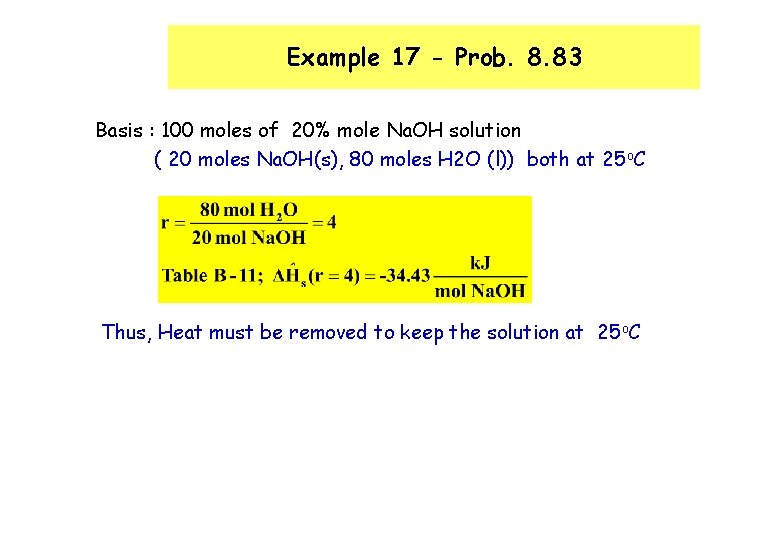
Example 17 - Prob. 8. 83 Basis : 100 moles of 20% mole Na. OH solution ( 20 moles Na. OH(s), 80 moles H 2 O (l)) both at 25 o. C Thus, Heat must be removed to keep the solution at 25 o. C

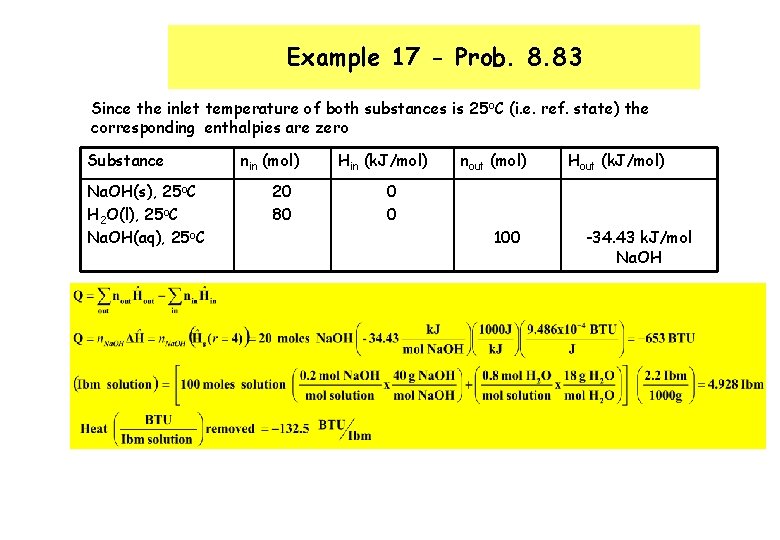
Example 17 - Prob. 8. 83 Since the inlet temperature of both substances is 25 o. C (i. e. ref. state) the corresponding enthalpies are zero Substance Na. OH(s), 25 o. C H 2 O(l), 25 o. C Na. OH(aq), 25 o. C nin (mol) 20 80 Hin (k. J/mol) nout (mol) Hout (k. J/mol) 0 0 100 -34. 43 k. J/mol Na. OH

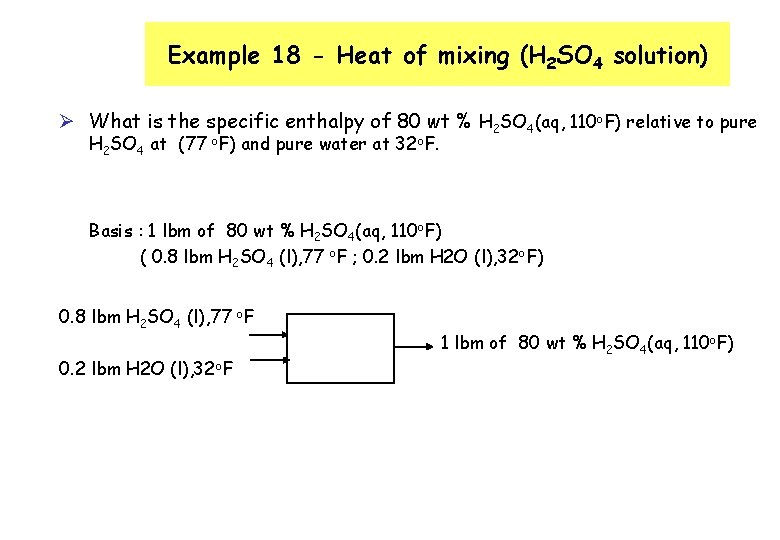
Example 18 - Heat of mixing (H 2 SO 4 solution) Ø What is the specific enthalpy of 80 wt % H 2 SO 4(aq, 110 o. F) relative to pure H 2 SO 4 at (77 o. F) and pure water at 32 o. F. Basis : 1 lbm of 80 wt % H 2 SO 4(aq, 110 o. F) ( 0. 8 lbm H 2 SO 4 (l), 77 o. F ; 0. 2 lbm H 2 O (l), 32 o. F) 0. 8 lbm H 2 SO 4 (l), 77 o. F 0. 2 lbm H 2 O (l), 32 o. F 1 lbm of 80 wt % H 2 SO 4(aq, 110 o. F)

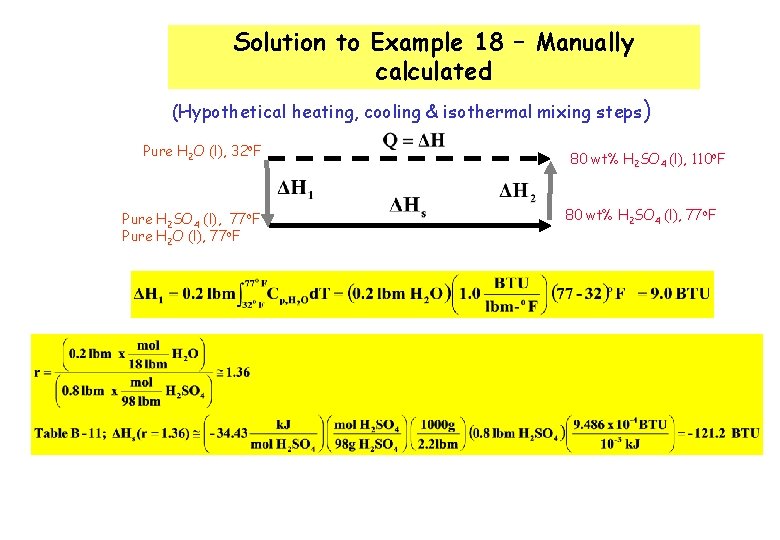
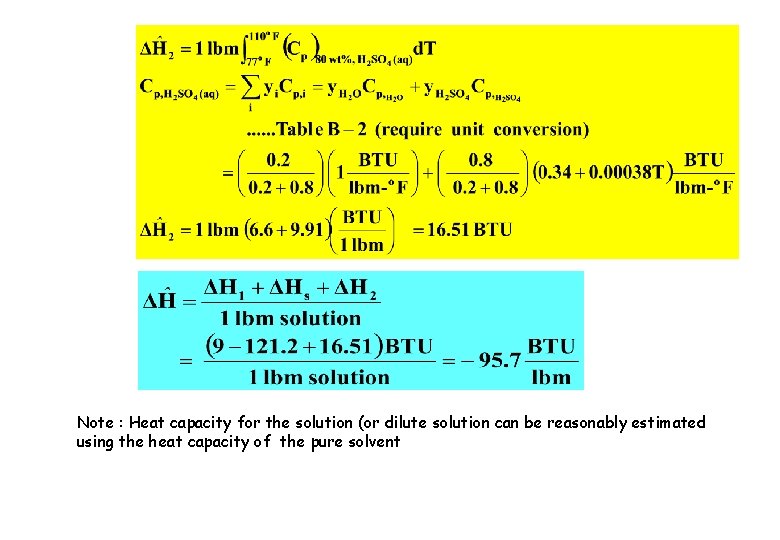
Solution to Example 18 – Manually calculated (Hypothetical heating, cooling & isothermal mixing steps ) Pure H 2 O (l), 32 o. F Pure H 2 SO 4 (l), 77 o. F Pure H 2 O (l), 77 o. F 80 wt% H 2 SO 4 (l), 110 o. F 80 wt% H 2 SO 4 (l), 77 o. F

Note : Heat capacity for the solution (or dilute solution can be reasonably estimated using the heat capacity of the pure solvent

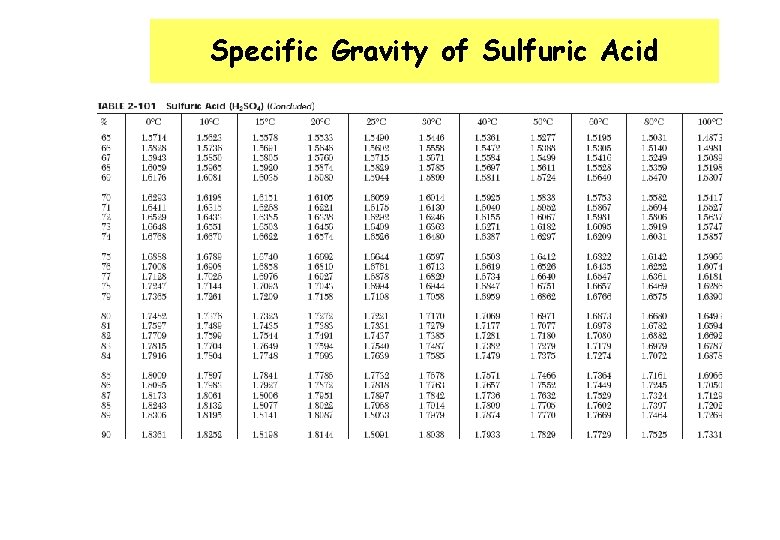
Specific Gravity of Sulfuric Acid