MIXING AND SEPARATING CHEMISTRY WHAT IS A MIXTURE






















- Slides: 26

MIXING AND SEPARATING CHEMISTRY

WHAT IS A MIXTURE? • COMBINATIONS OF TWO OR MORE SUBSTANCES (START A GLOSSARY)

MIXTURES IN EVERY DAY LIFE…… ADD AN EXAMPLE TO YOUR

CEMENT

BOLTE BRIDGEMELBOURNE

• THE BOLTE BRIDGE IS AN EYECATCHING STRUCTURE CONNECTING FREEWAYS ON THE EDGE OF THE CITY OF MELBOURNE. • OVER 50 000 TONNES OF GOLIATH CEMENT WAS USED FOR THE BRIDGE. • THE BRIDGE IS SUPPORTED BY UNDERWATER FOUNDATIONS AND OVER 3500 SECTIONS OF ROAD WIDE ENOUGH FOR SIX LANES OF TRAFFIC. • THE CEMENT IS A MIXTURE OF PURE SUBSTANCES, MOST NATURALLY OCCURRING, THAT WERE MIXED IN JUST THE RIGHT AMOUNTS TO GET THE REQUIRED BALANCE OF PHYSICAL PROPERTIES.

OVER TO YOU! 1. WHAT IS A MIXTURE? 2. WHY IS IT IMPORTANT TO MIX THE RIGHT INGRE DIENTS IN THE RIGHT AMOUNTS? 3. GOLIATH CEMENT IS MADE OF ABOUT SEVEN DI FFERENT PURE SUBSTANCES. WHAT COULD THEY BE?

PURE SUBSTANCES • SOMETHING THAT IS NOT MIXED OR COMBINED WITH ANYTHING ELSE (ADD TO GLOSSARY)

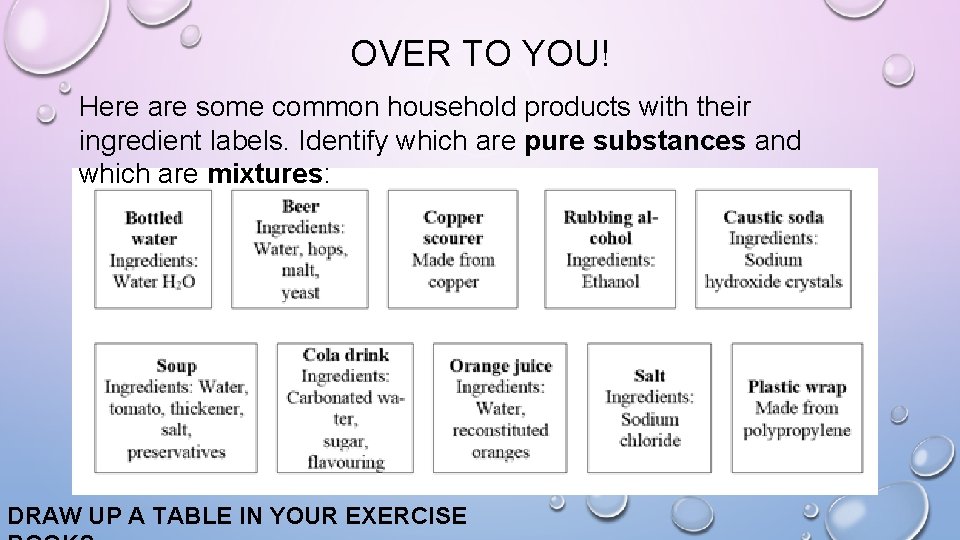
OVER TO YOU! Here are some common household products with their ingredient labels. Identify which are pure substances and which are mixtures: DRAW UP A TABLE IN YOUR EXERCISE

TABLE PURE SUBSTANCES MIXTURES

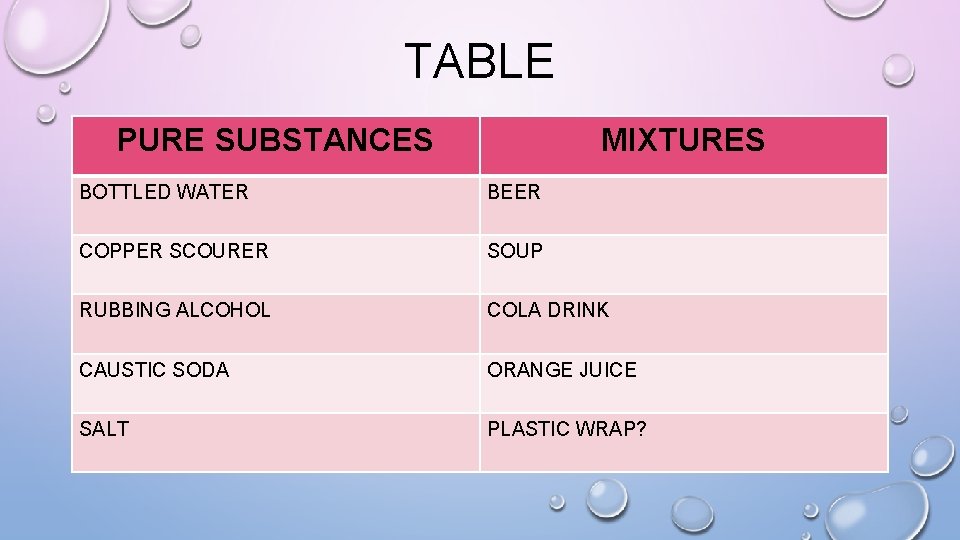
TABLE PURE SUBSTANCES MIXTURES BOTTLED WATER BEER COPPER SCOURER SOUP RUBBING ALCOHOL COLA DRINK CAUSTIC SODA ORANGE JUICE SALT PLASTIC WRAP?

MIXTURES SUMMARY • Most of the substances we use every day are mixtures. • Mixtures are combinations of two or more substances. • Most mixtures look like just one substance because the pa rticles they are made from are so small. • There are so many possible combinations of mixtures, eac h with different properties and purposes. • For this reason, scientists have grouped mixtures accordin g to what they are made from and their behaviour.

WHAT ARE THESE MIXTURES MADE UP OF?

TYPES OF MIXTURES Suspensions: cloudy liquids that contain insoluble particles (Add to Glossary) • Dirty water is a type of mixture called a suspension. • Suspensions will often need to be shaken or stirred before use, to spread the sediment through the liquid again.

A snow dome can be described as a suspension, with the ‘snow’ being suspended in the water for a short time before it falls to the bottom of the dome to form

PRACTICAL ACTIVITY Dirty water What you need: soil, water, jar with screw top lid 1. Put some soil and water in the jar to create a w atery mixture. 2. Screw the lid on tightly, then shake the jar. Questions: What happens to the soil particles? Does anything float on the water? How can you explain the behaviour of this mixture?

TYPES OF MIXTURES Colloids: types of mixtures that always looks cloudy because clumps of insoluble particles remain suspended throughout it-they don’t settle as sediment (Add to Glossary) • When two or more substances are mixed, they don’t always sep arate out with time. Suspensions that don’t separate easily are referred to as colloids. • These can be formed by a solid in a liquid but can also involve only liquids, only gases or even a solid suspended in a gas. • The word ‘colloid’ comes from the Greek word kolla which means ‘glue’. You can think of a colloid as a substance being ‘stuck’—

COLLOIDS Milk is a colloid because it contains many substances suspended in what is mainly water. Fog is a colloid because it is ai r containing suspended liqu idparticles.

TYPES OF MIXTURES Emulsions: stable mixtures of two or more liquids, e. g. milk (Add to Glossary) • An emulsion is a colloid of two or more liquids. Usually, one li quid is the ‘base’ and the other is broken into tiny droplets spread throughout the ‘base’ liquid. Milk is an example of an emulsion, with tiny droplets of fats and oils spread throughout the base, which is water. • In some cases, when mixtures like this are left to settle, the tiny

TYPES OF MIXTURES Solutions: liquid made up of a solvent with a solute dissolved in it Solvent: any liquid that dissolves another substance or substances Solute: dissolves in a liquid (solvent) Copy out definitions into Glossary!

SOLUTIONS • Sometimes water contains salts that affect its taste and behaviour. • This type of water is an example of a mixture that doesn’t separate by itself-a solution. • Spread evenly throughout solutions are particles so tiny that the solution looks transparent. • We say that sugar can dissolve in water to form a solution. • A substance that is able to dissolve in a liquid is considered to be soluble, while one that cannot is insoluble. • The substance dissolving is called the solute, while the liquid into which it dissolves is called the solvent. • Sometimes it is necessary to help a solute to dissolve. • Warming is the most

MORE DEFINITIONS Soluble: dissolves very easily in water e. g. Salt and Sugar Insoluble: does not dissolve


WORKING WITH SOLUTIONS • You have seen that a solution is a solute dissolved in a solvent. • Solutions can be compared in terms of their: Concentration: how much solute is in the solvent. If just a little solu te is dissolved, the solution is described as dilute (low concentration). If a lot of a solute is dissolved, then the solution is described as concentrated (high concentration). • It is only possible to dissolve a certain amount of a particular solu te in a solvent. If no more solute can dissolve into a solution, the solution is described as saturated-it cannot dissolve any more. • We often work with solutions. By adding solutes to pure liquids, t he properties of the pure liquids may be changed.

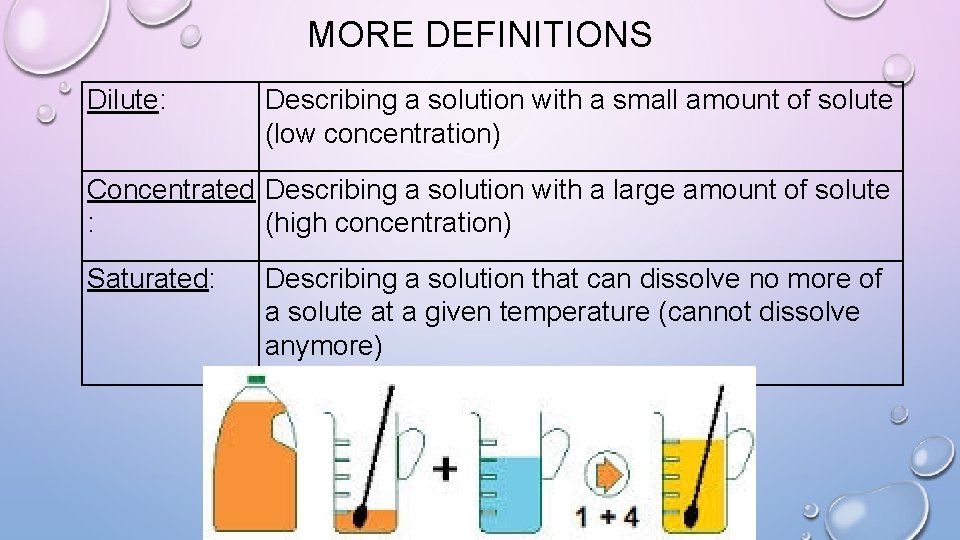
MORE DEFINITIONS Dilute: Describing a solution with a small amount of solute (low concentration) Concentrated Describing a solution with a large amount of solute : (high concentration) Saturated: Describing a solution that can dissolve no more of a solute at a given temperature (cannot dissolve anymore)

LEARNING VOCABULARY 1. CREATE YOUR OWN WORD SEARCH -USE THE DEFINITIONS OF THE WORDS AS CLUES -SWAP WITH A FRIEND 2. USING YOUR CUE-CARDS TEST A PARTNER