MIX IT UP Mixtures Solutions and Solubility how

MIX IT UP! Mixtures, Solutions and Solubility: how mixed up is this crazy world?

What do you notice about these substances?

OBJECTIVES ·Learner will: • Demonstrate an understanding of the physical nature of science by classifying substances as elements, compounds, or mixtures · Demonstrate an understanding of the physical nature of science by distinguishing among solvents, solutes, and solutions (PS. 5. 7. 6) (PS. 5. 7. 7)

MIXTURES Combination of substances that: �Are not bonded together �Can be separated by physical means
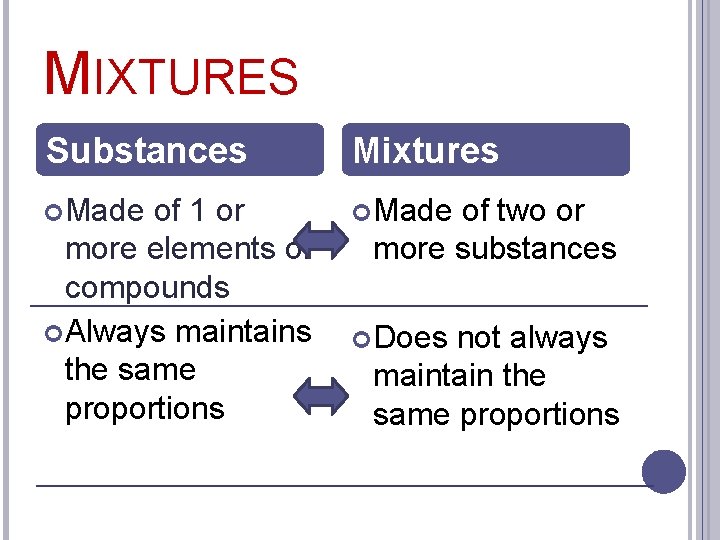
MIXTURES Substances Mixtures Made of 1 or more elements or compounds Always maintains the same proportions of two or more substances Does not always maintain the same proportions

MIXTURES Substances Mixtures Chemically Not combined Cannot be separated by physical means chemically combined Can be separated by physical means

MIXTURES Two types �Heterogeneous �Homogeneous
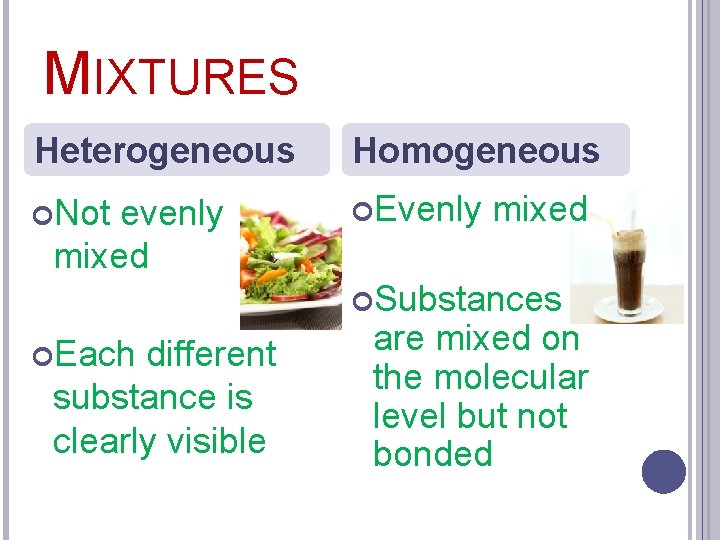
MIXTURES Heterogeneous Homogeneous Not Evenly evenly mixed Substances Each different substance is clearly visible are mixed on the molecular level but not bonded

SOLUTIONS Solution �Homogeneous mixture

SOLUTIONS Two parts �Solute- dissolved �Solvent- dissolves the solute

SOLUTIONS Liquid solutions �Can form with solids, liquids or gases Gaseous solutions �Forms only with other gases Solid solutions �Can form with solids, liquids or gases

SOLUTIONS Precipitate �Solid that comes back out of a solution �Example: stalactites and stalagmites

This is a natural phenomena that occurs in our own backyard! BLANCHARD SPRINGS CAVERNS
OBJECTIVES Learner will: · Understand the physical nature or science by observing the effects of variables on solubility rates · Apply the knowledge of the physical nature of science by interpreting solubility graphs (PS. 5. 7. 8) (PS. 5. 7. 9)

SOLUBILITY Water is called the universal solvent because it can dissolve so many substances
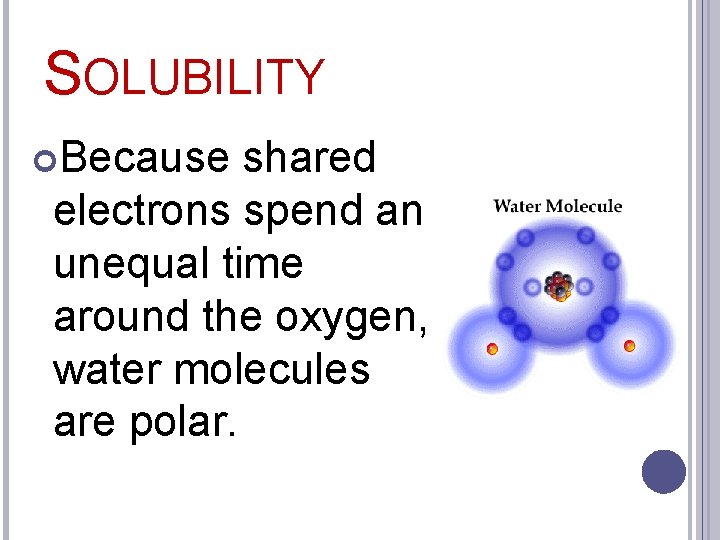
SOLUBILITY Because shared electrons spend an unequal time around the oxygen, water molecules are polar.
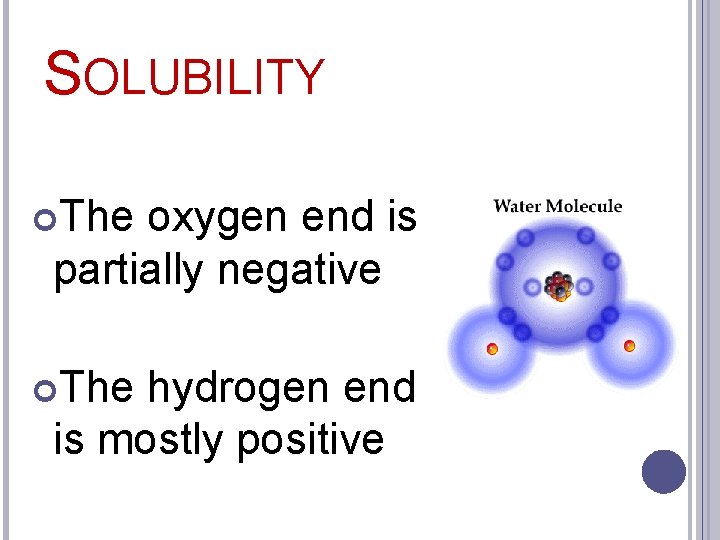
SOLUBILITY The oxygen end is partially negative The hydrogen end is mostly positive
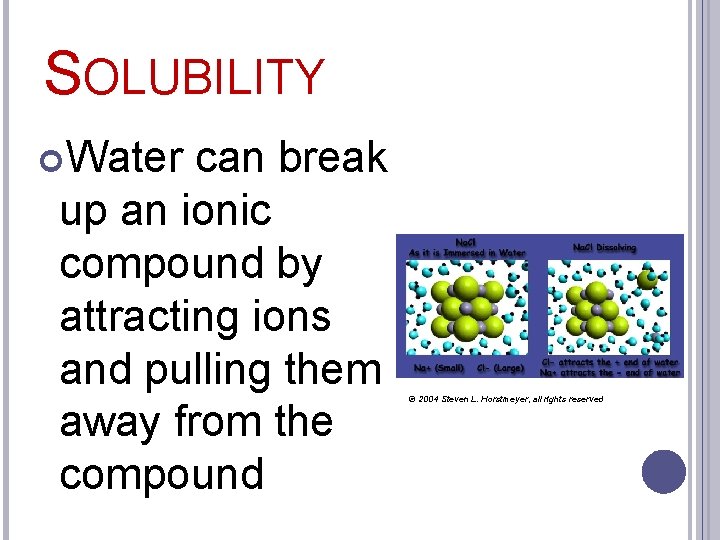
SOLUBILITY Water can break up an ionic compound by attracting ions and pulling them away from the compound © 2004 Steven L. Horstmeyer, all rights reserved

SOLUBILITY Water can also break up larger molecules by slipping in between them and attracting the polar areas © 2004, Arthur L. Buikema, Jr. All rights reserved.

SOLUBILITY Like-dissolves-like �Polar – polar �Non-polar – non-polar �Complex molecules may dissolve both

SOLUBILITY Polar �Dissolves ionic substances �Breaks apart large molecules

SOLUBILITY Non-polar �Dissolves covalent substances

SOLUBILITY Solubility �Measurement of solute dissolved in solvent

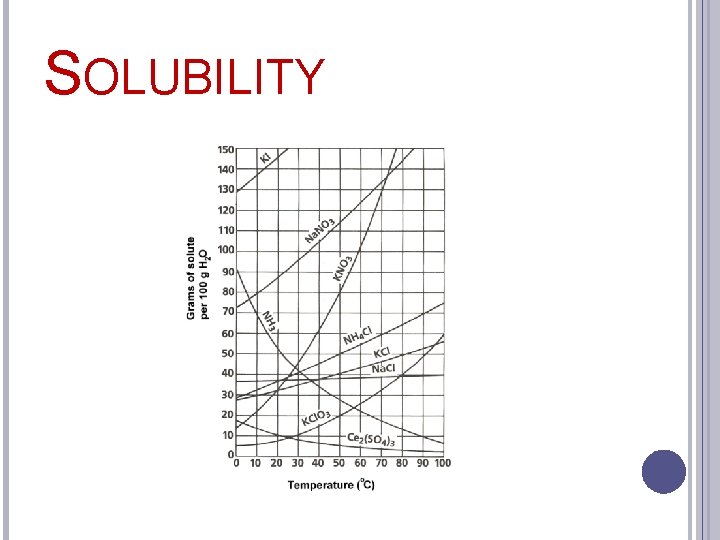
SOLUBILITY Temperature �Factor that increases solubility

SOLUBILITY Temperature �Liquid-solid solutions Solubility increases with rise in temperature

SOLUBILITY Temperature �Liquid-gas solutions Solubility decreases with rise in temperature
SOLUBILITY

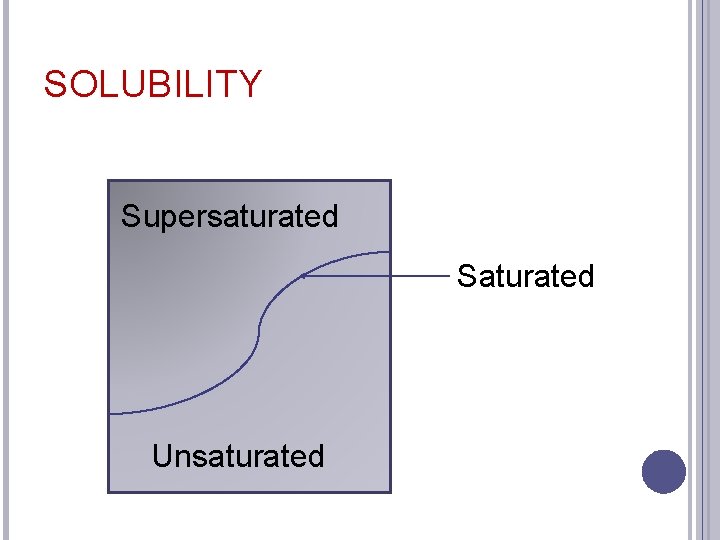
SOLUBILITY Saturated �A solution holds as much solute as it can at a given temperature
SOLUBILITY Supersaturated Saturated Unsaturated
OBJECTIVES Learner will: · Demonstrate an understanding of the physical nature of science by distinguishing among solvents, solutes, and solutions (PS. 5. 7. 7)

SOLUBILITY Rate of dissolving �Increases with temperature �Increases with agitation

SOLUBILITY Concentration �Amount solute compared to amount of solvent
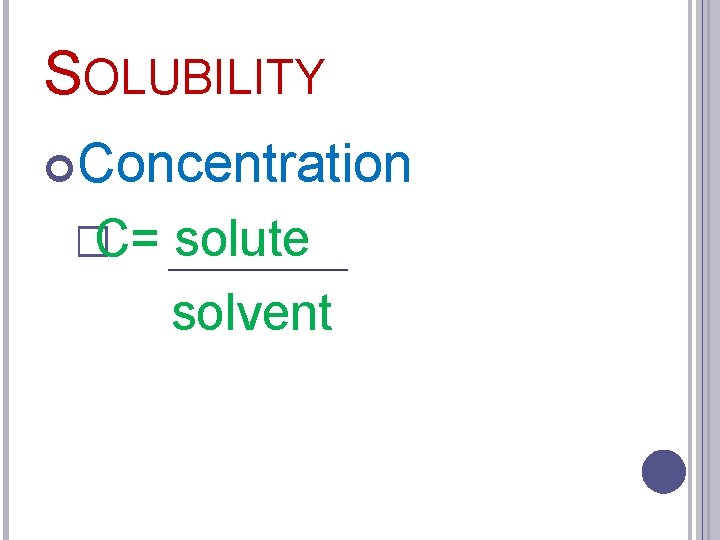
SOLUBILITY Concentration �C= solute solvent

SOLUBILITY Solutes �Effect physical properties

OBJECTIVES Learner will: ·Apply knowledge of the physical nature of science by demonstrating techniques forming and separating mixtures • Investigate breakthroughs related to mixtures (PS. 5. 7. 5) (PS. 5. 7. 10)

FORMING MIXTURES Two ways �Mixing �Dissolving
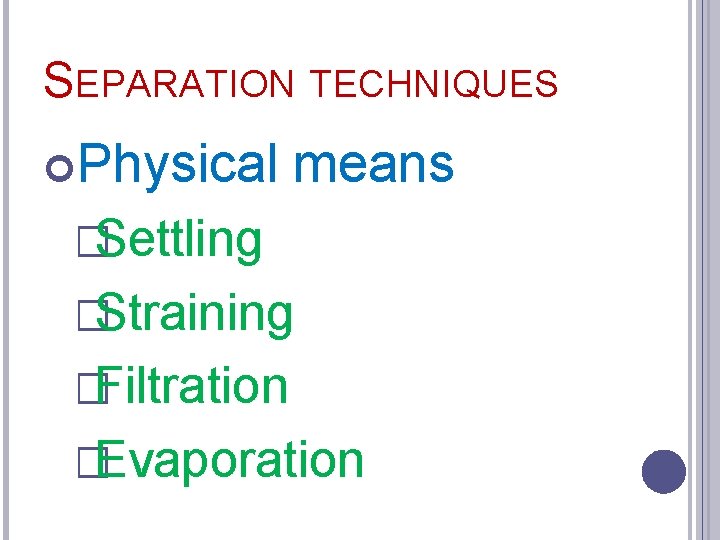
SEPARATION TECHNIQUES Physical means �Settling �Straining �Filtration �Evaporation

SEPARATION TECHNIQUES Physical means �Chromatography �Electrophorensis �Light and electromagnetic spectrum
- Slides: 38