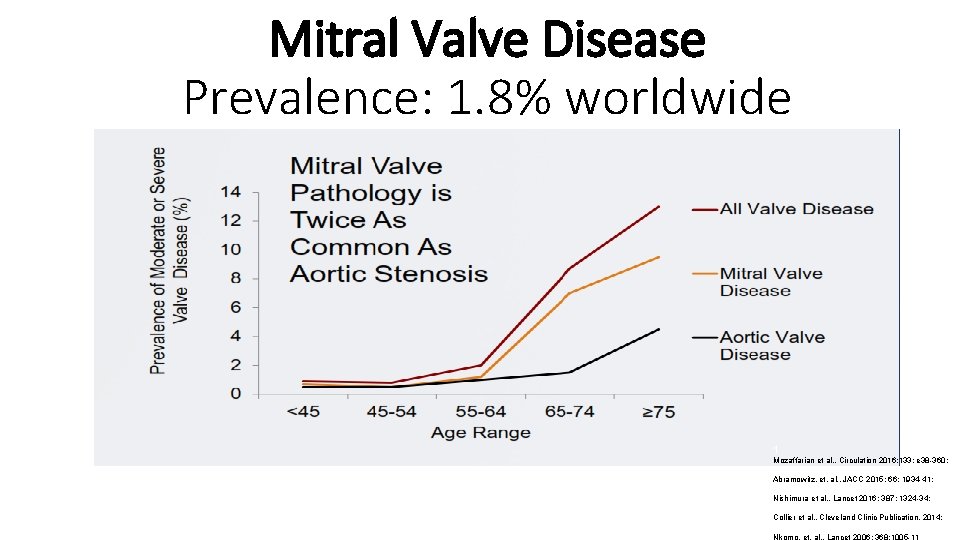
Mitral Valve Disease Prevalence 1 8 worldwide 1






- Slides: 59


Mitral Valve Disease Prevalence: 1. 8% worldwide 1 Mozaffarian et al. , Circulation 2016; 133: e 38 -360; Abramowitz, et. al. , JACC 2015; 66: 1934 -41; Nishimura et al. , Lancet 2016; 387: 1324 -34; Collier et al. , Cleveland Clinic Publication, 2014; Nkomo, et. al. , Lancet 2006; 368: 1005 -11

ü Mitral regurgitation (MR) is one of the most common heart valve disorders ü If left untreated it leads to progressive LV dysfunction, heart failure, and death. ü Surgical repair or replacement is the established treatment for degenerative MR. ü Patients with associated comorbidities or LV dysfunction put them at very high surgical risk. ü Need exists for less invasive, safer options for selected patients

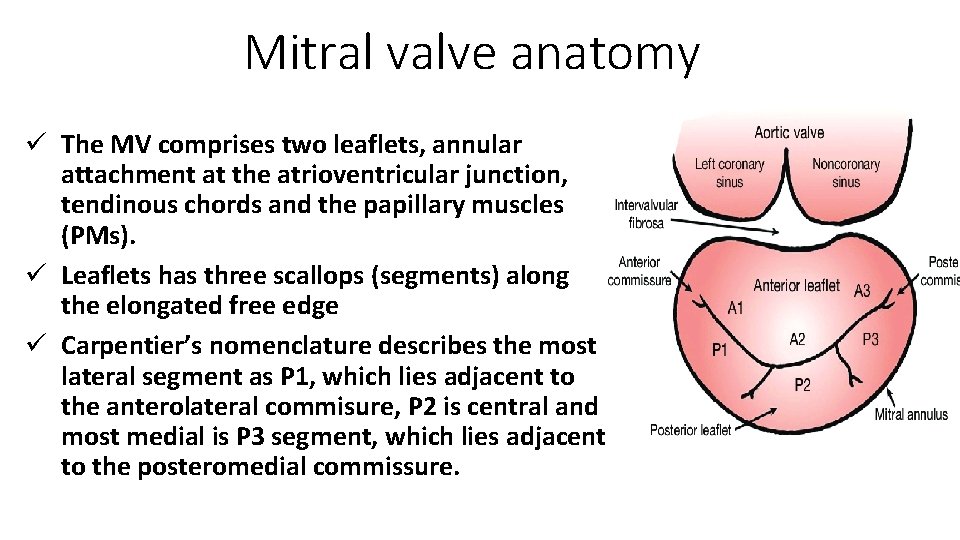
Mitral valve anatomy ü The MV comprises two leaflets, annular attachment at the atrioventricular junction, tendinous chords and the papillary muscles (PMs). ü Leaflets has three scallops (segments) along the elongated free edge ü Carpentier’s nomenclature describes the most lateral segment as P 1, which lies adjacent to the anterolateral commisure, P 2 is central and most medial is P 3 segment, which lies adjacent to the posteromedial commissure.

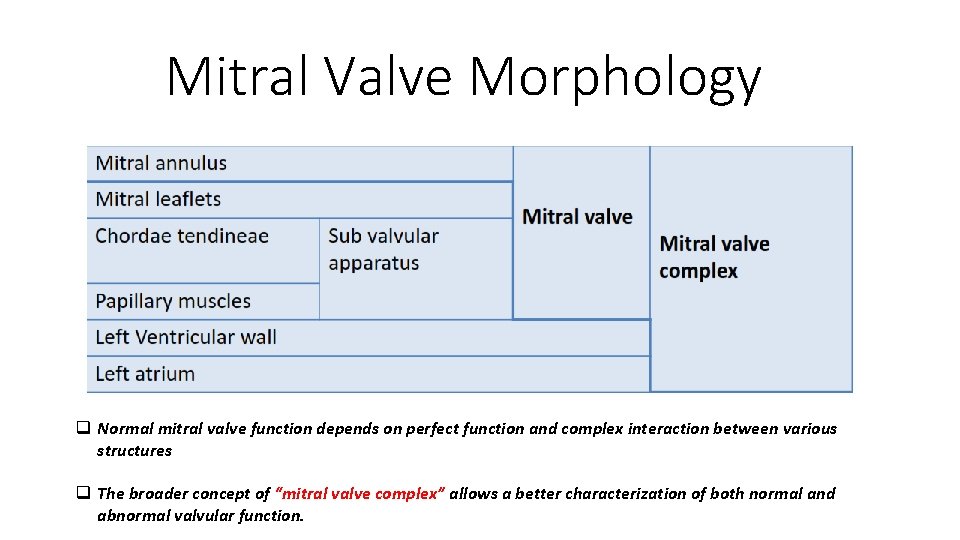
Mitral Valve Morphology q Normal mitral valve function depends on perfect function and complex interaction between various structures q The broader concept of “mitral valve complex” allows a better characterization of both normal and abnormal valvular function.


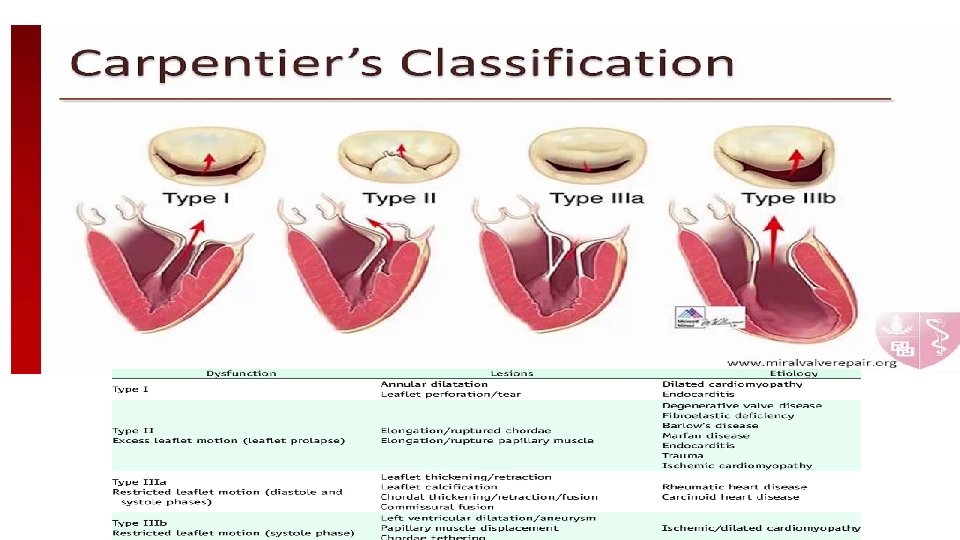
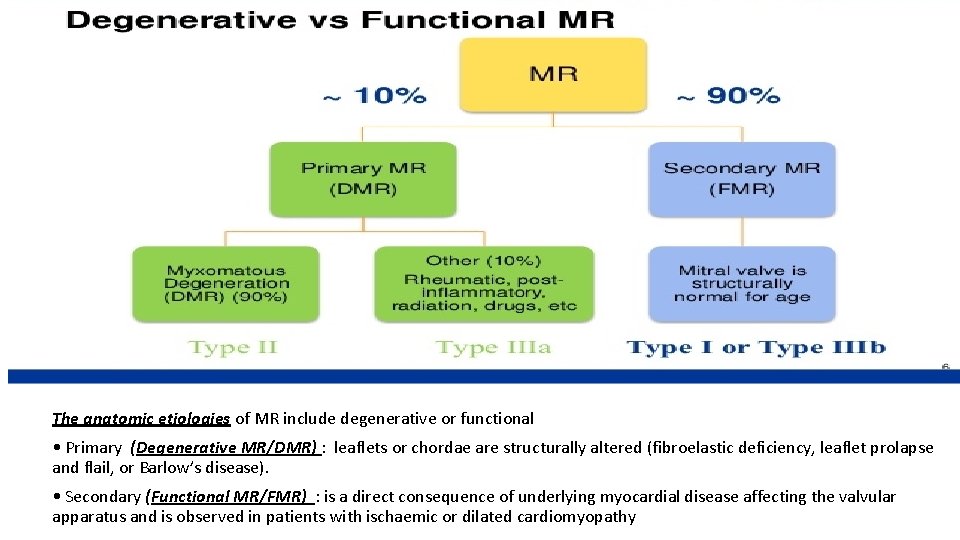
Etiology of MR The anatomic etiologies of MR include degenerative or functional • Primary (Degenerative MR/DMR) : leaflets or chordae are structurally altered (fibroelastic deficiency, leaflet prolapse and flail, or Barlow’s disease). • Secondary (Functional MR/FMR) : is a direct consequence of underlying myocardial disease affecting the valvular apparatus and is observed in patients with ischaemic or dilated cardiomyopathy

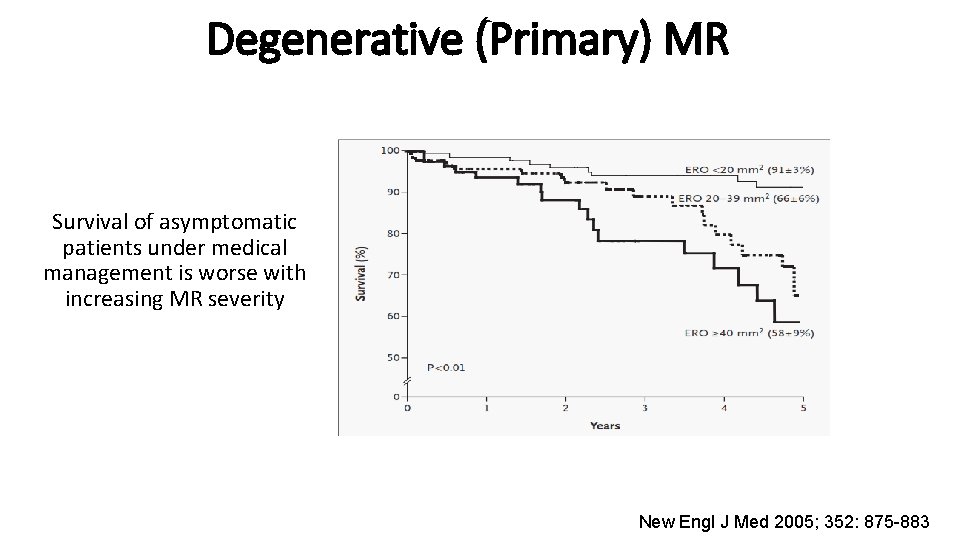
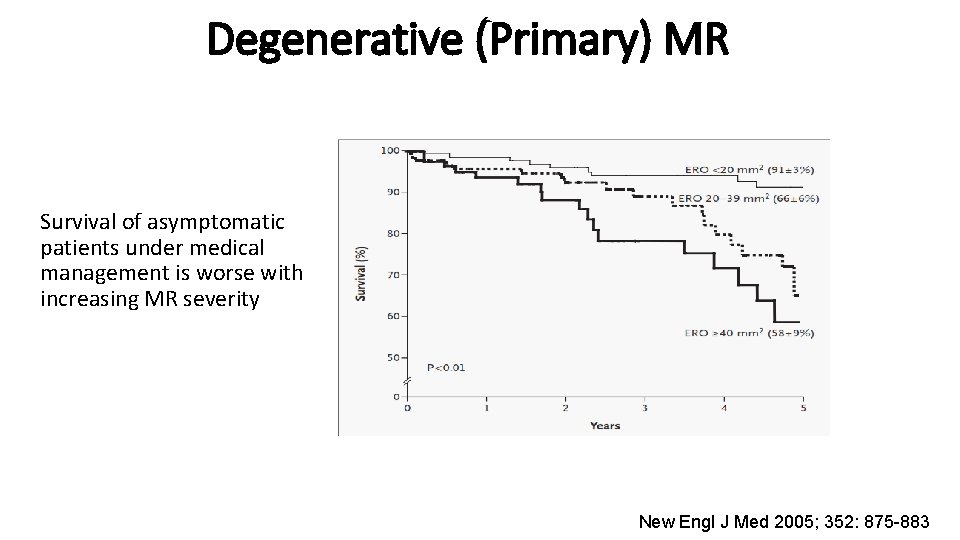
Degenerative (Primary) MR Survival of asymptomatic patients under medical management is worse with increasing MR severity New Engl J Med 2005; 352: 875 -883

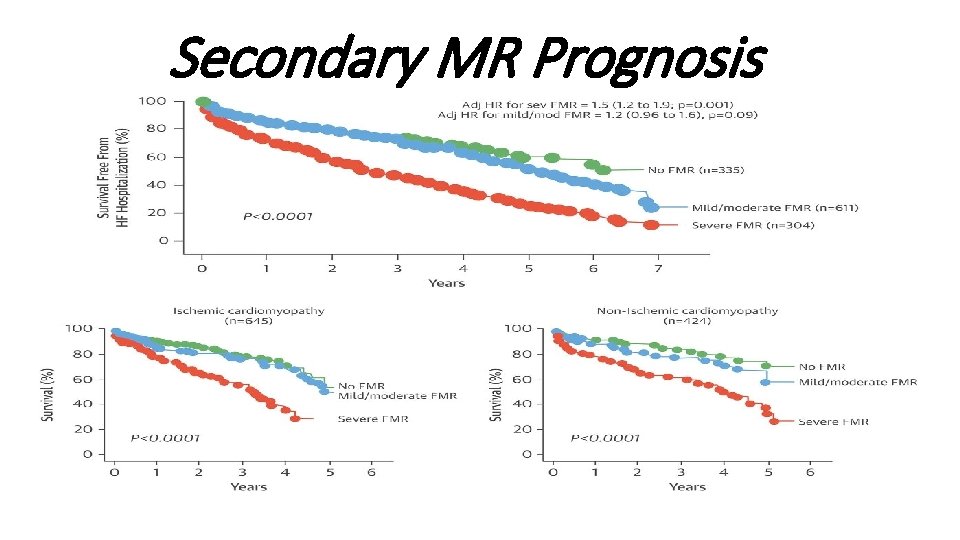
Secondary MR Prognosis

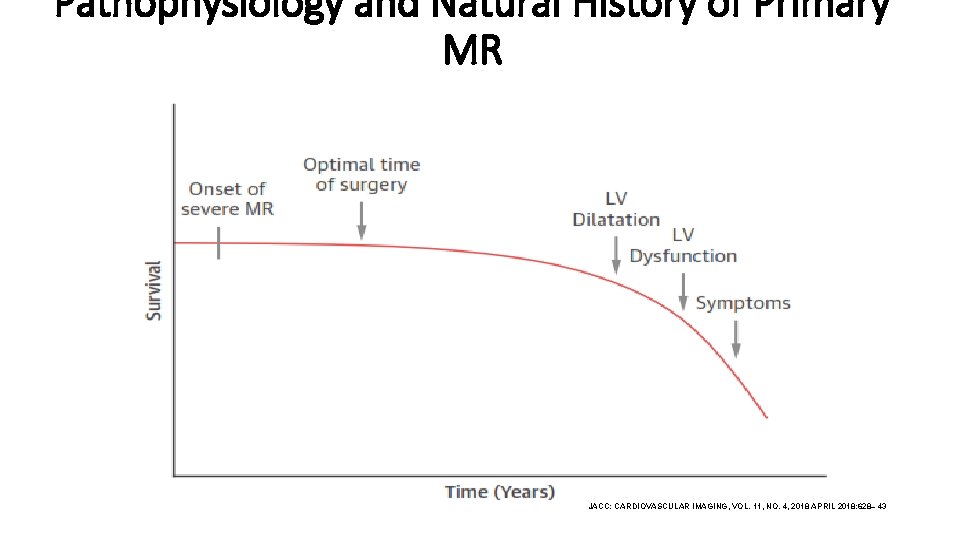
Pathophysiology and Natural History of Primary MR JACC: CARDIOVASCULAR IMAGING, VOL. 11, NO. 4, 2018 APRIL 2018: 628– 43

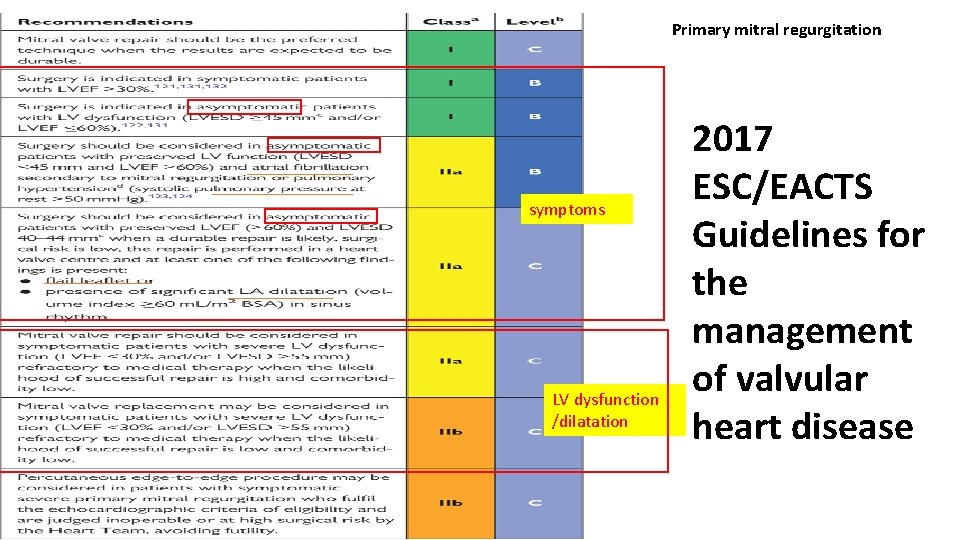
Primary mitral regurgitation symptoms LV dysfunction /dilatation 2017 ESC/EACTS Guidelines for the management of valvular heart disease

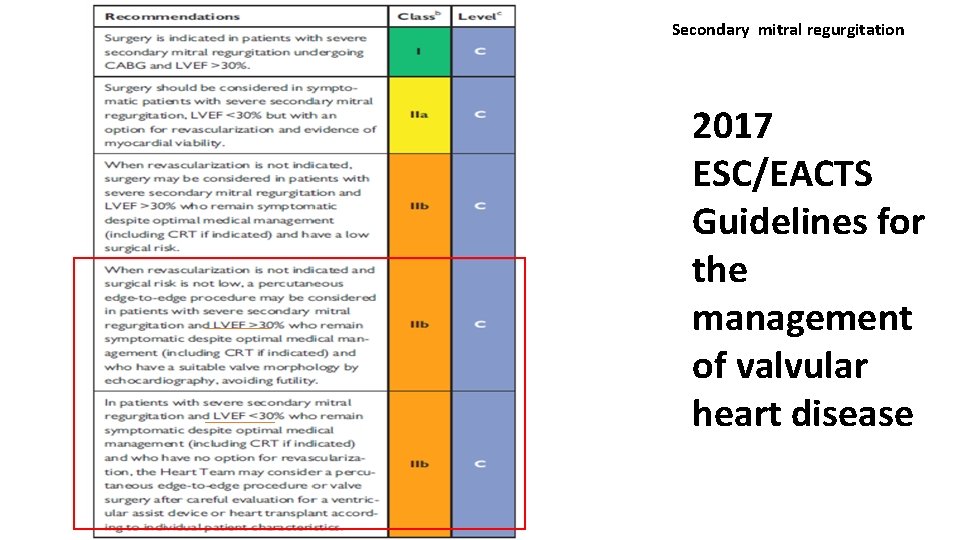
Secondary mitral regurgitation 2017 ESC/EACTS Guidelines for the management of valvular heart disease

Degenerative (Primary) MR Survival of asymptomatic patients under medical management is worse with increasing MR severity New Engl J Med 2005; 352: 875 -883

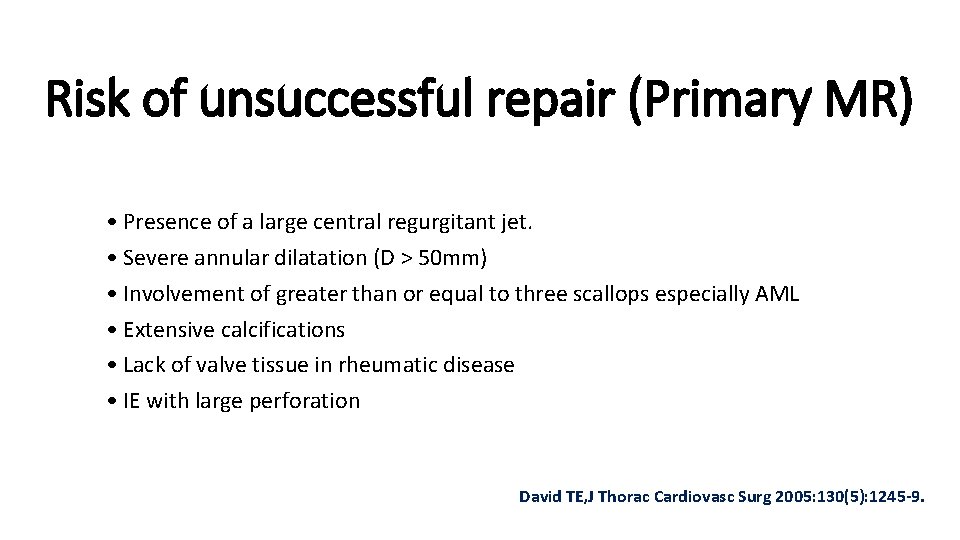
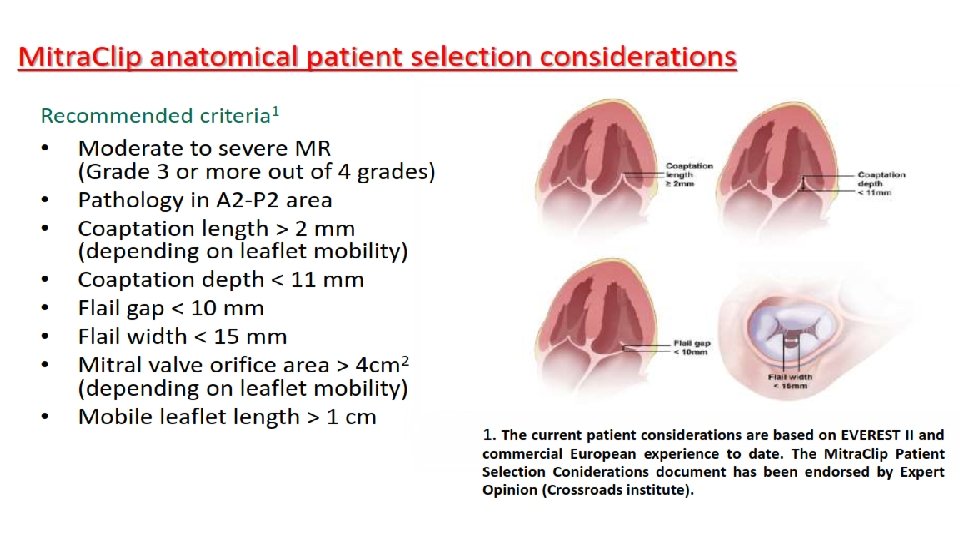
Risk of unsuccessful repair (Primary MR) • Presence of a large central regurgitant jet. • Severe annular dilatation (D > 50 mm) • Involvement of greater than or equal to three scallops especially AML • Extensive calcifications • Lack of valve tissue in rheumatic disease • IE with large perforation David TE, J Thorac Cardiovasc Surg 2005: 130(5): 1245 -9.

Ideal patient for Percutaneous Mitral valve repair • Difficult to precisely define the subgroups of patients who will benefit from repair • Pathology of MR to be known • Symptomatic severe MR • Not candidates for surgical correction • Preference of a less invasive approach without need of a CABG • Comorbidities conferring a high surgical risk. • Life expectancy more than 1 yr.

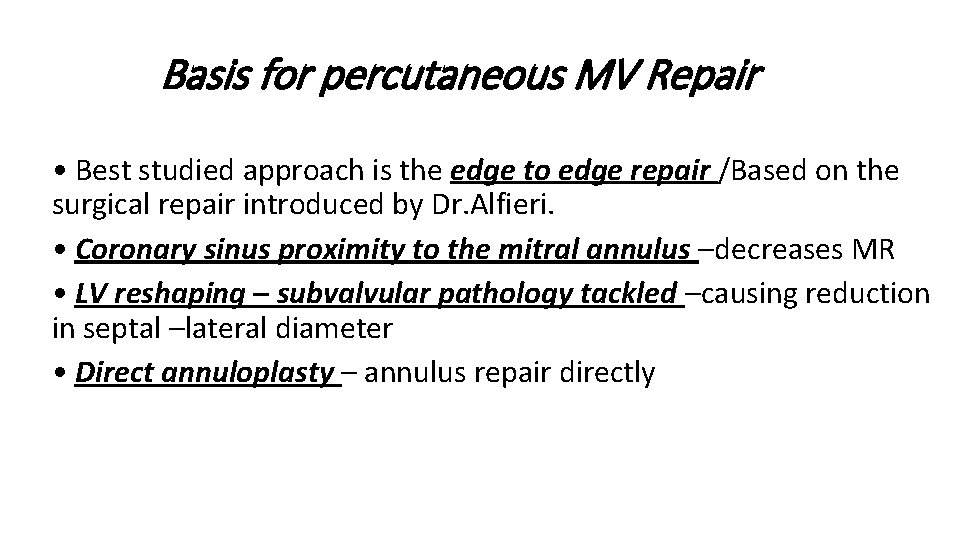
Basis for percutaneous MV Repair • Best studied approach is the edge to edge repair /Based on the surgical repair introduced by Dr. Alfieri. • Coronary sinus proximity to the mitral annulus –decreases MR • LV reshaping – subvalvular pathology tackled –causing reduction in septal –lateral diameter • Direct annuloplasty – annulus repair directly

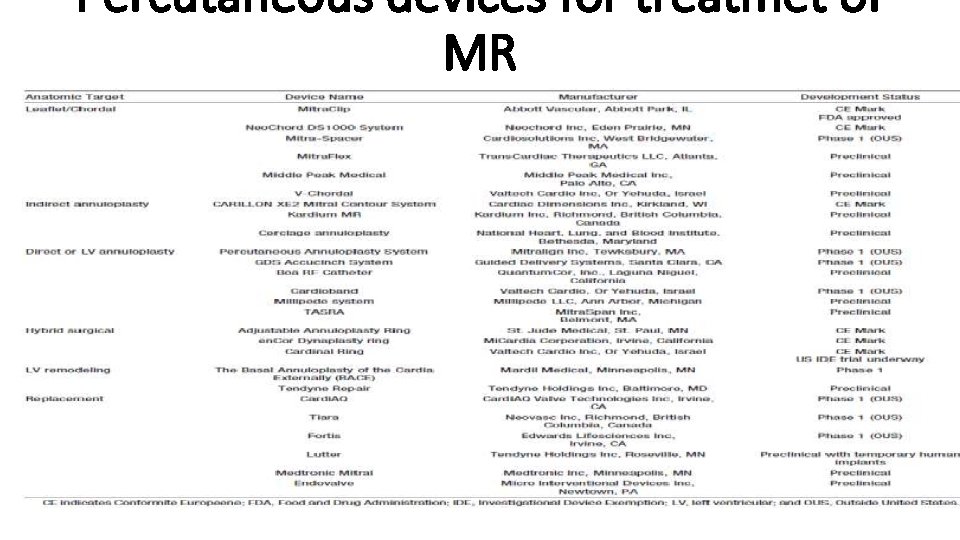
Percutaneous devices for treatmet of MR

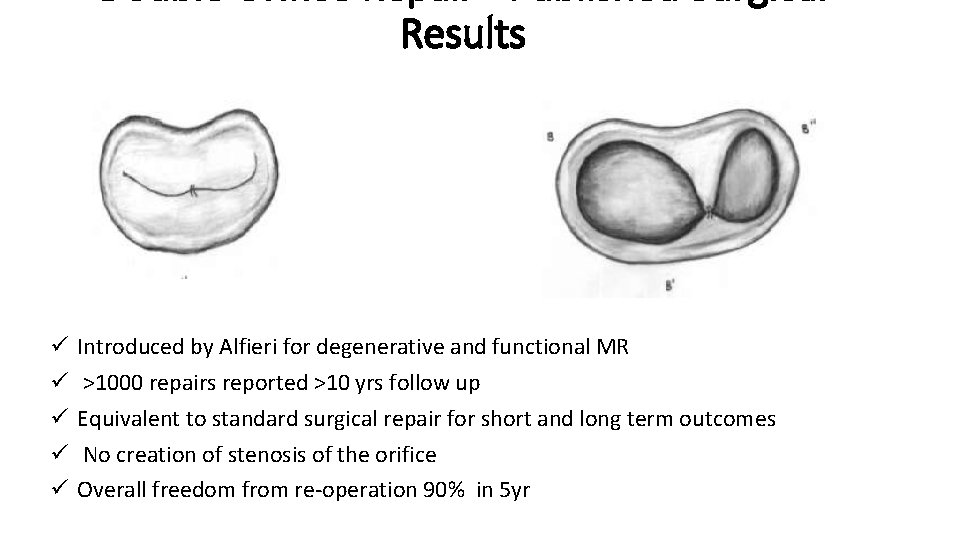
Results ü ü ü Introduced by Alfieri for degenerative and functional MR >1000 repairs reported >10 yrs follow up Equivalent to standard surgical repair for short and long term outcomes No creation of stenosis of the orifice Overall freedom from re-operation 90% in 5 yr

HISTORY AND DEVELOPMENT OF MITRAL CLIP ü The development of the Mitra. Clip goes back in 1998, when interventional cardiologist Frederick St. Goar started experimenting with percutaneous techniques for MVR. ü The feasibility of percutaneous MVR with the Mitra. Clip device was first demonstrated in a animal model. ü The first human implant of a Mitra. Clip was performed in June 2003 by Dr. Jose Condado in Caracas Venezuela in a 48 -year-old woman with severe MR due to a bileaflet flail. ü The procedure was performed without complications and after successful clip deployment her MR decreased to <2+

Mitraclip for DMR ü In experienced centers, DMR is treated with surgical repair at low risk, long term durability of repair is achieved in the majority of patients ü 50% of Euro Heart Survey patients were not referred to surgery (Mirabel EHJ 2007) ü Age and comorbidity increase the risk of surgery (STS database, 2010) ü Surgery is not associated with improved Qo. L in most elderly patients (Maisano et al EJCTS 2009)

Mitraclip for FMR • Surgical treatment of FMR is associated with ü ü High hospital mortality High recurrence rate Long hospital stay Unproven survival benefit • Mitraclip for FMR ü Procedure more simple than for DMR ü Improvement of symptoms ü Failure does not modify the surgical option

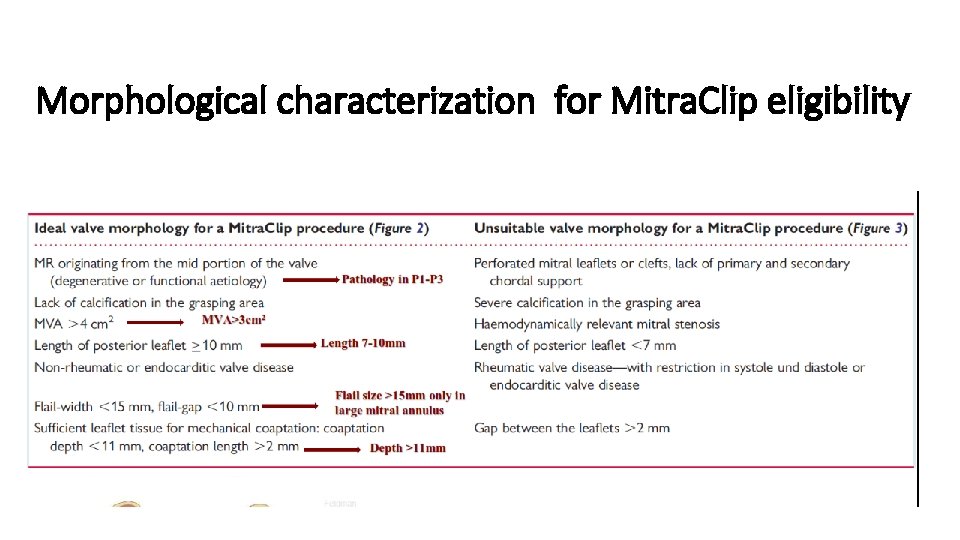
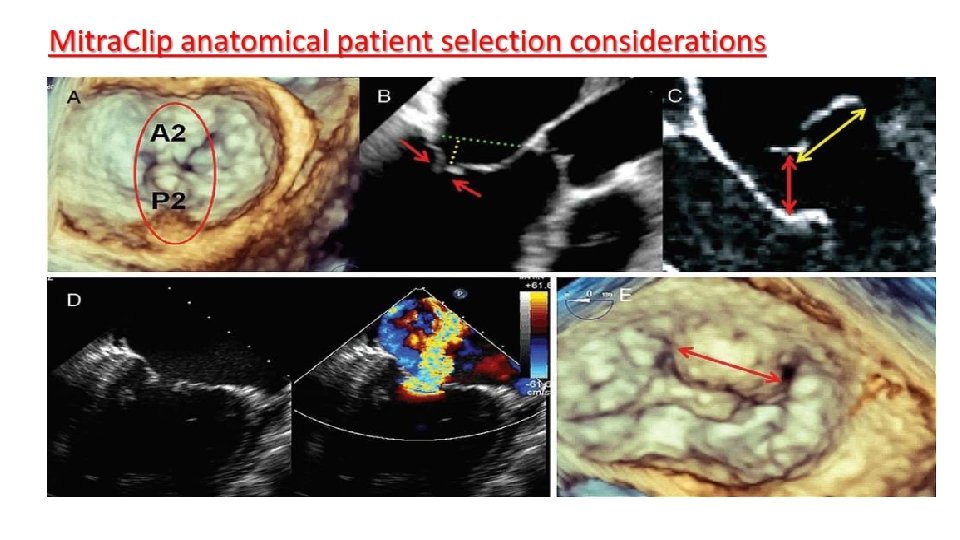
Morphological characterization for Mitra. Clip eligibility

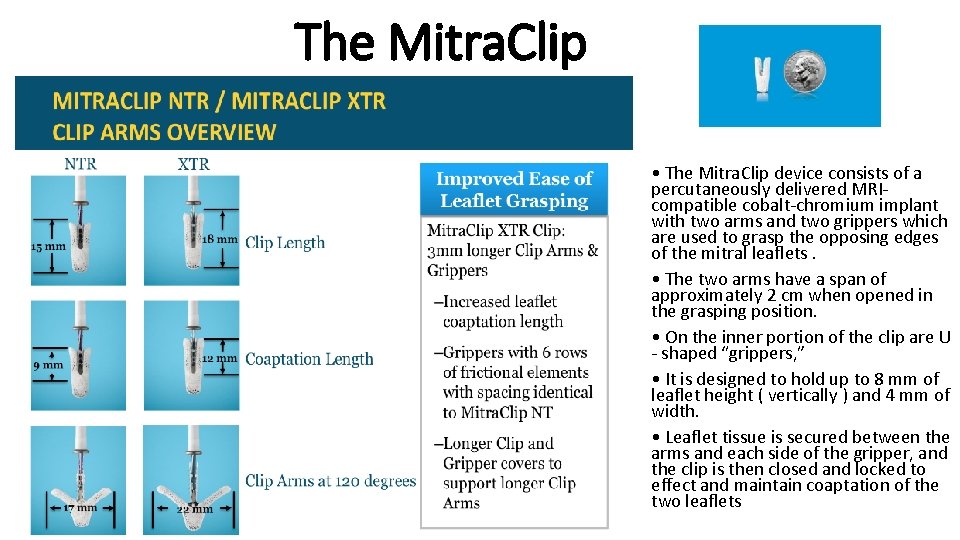
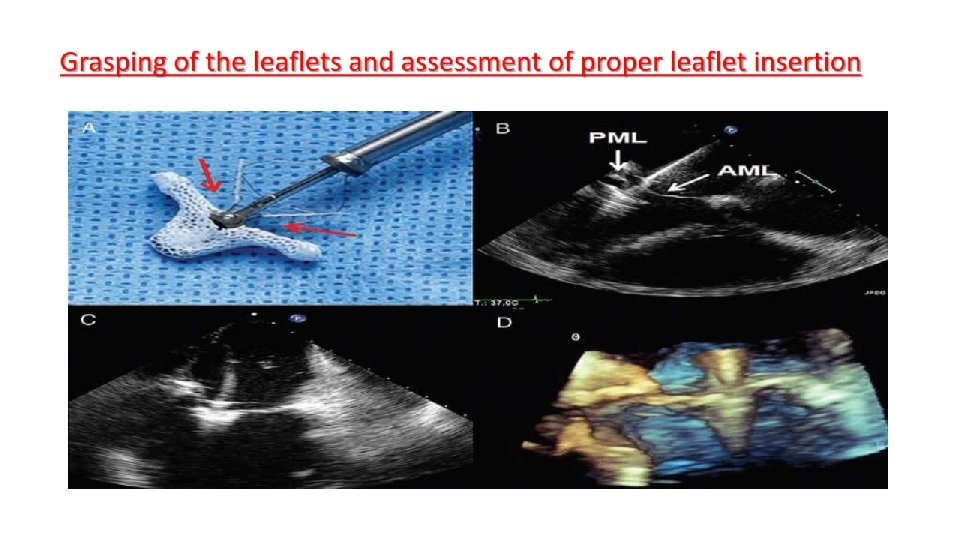
The Mitra. Clip • The Mitra. Clip device consists of a percutaneously delivered MRIcompatible cobalt-chromium implant with two arms and two grippers which are used to grasp the opposing edges of the mitral leaflets. • The two arms have a span of approximately 2 cm when opened in the grasping position. • On the inner portion of the clip are U - shaped “grippers, ” • It is designed to hold up to 8 mm of leaflet height ( vertically ) and 4 mm of width. • Leaflet tissue is secured between the arms and each side of the gripper, and the clip is then closed and locked to effect and maintain coaptation of the two leaflets

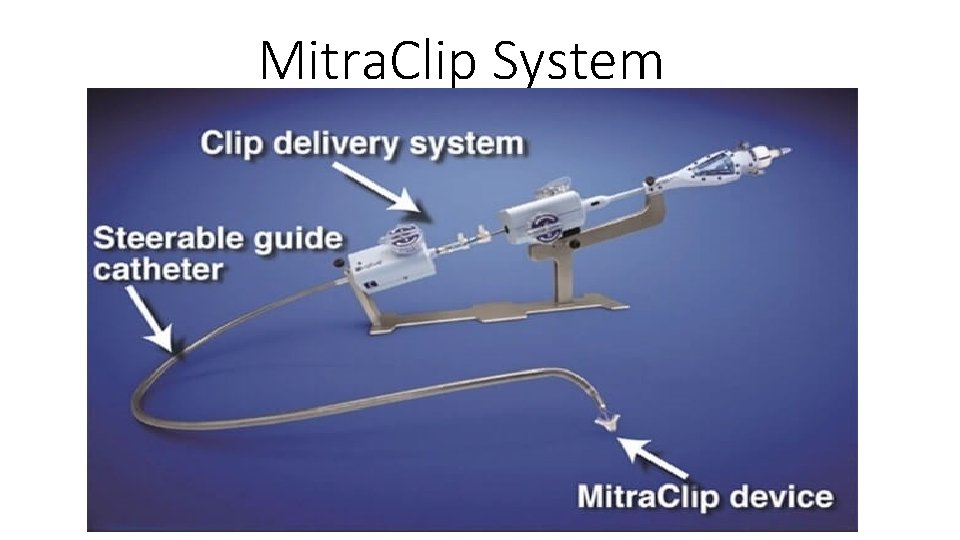
Mitra. Clip System







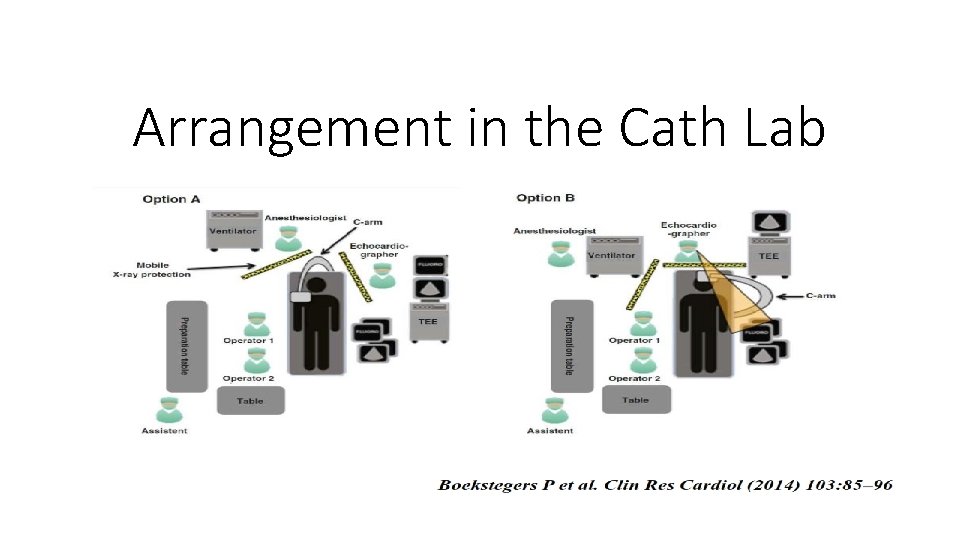
Arrangement in the Cath Lab

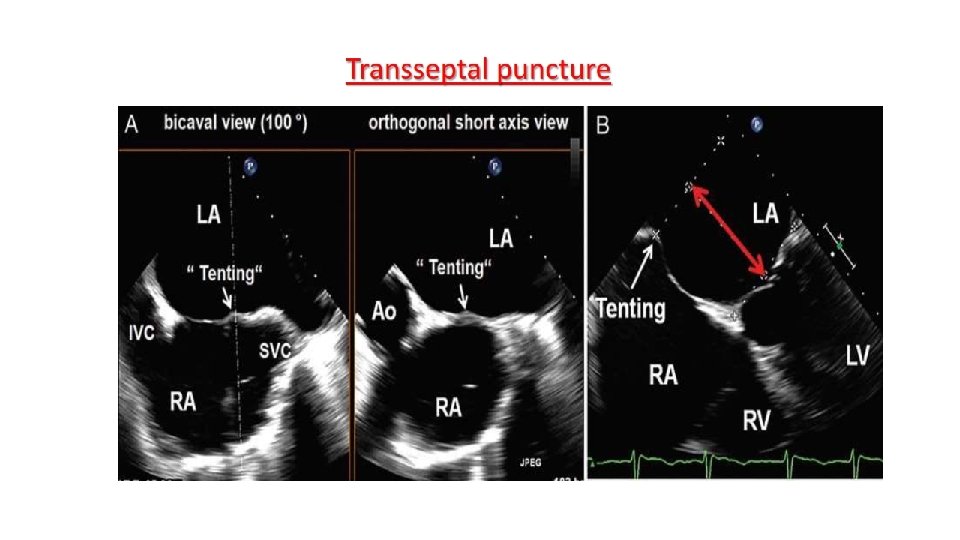
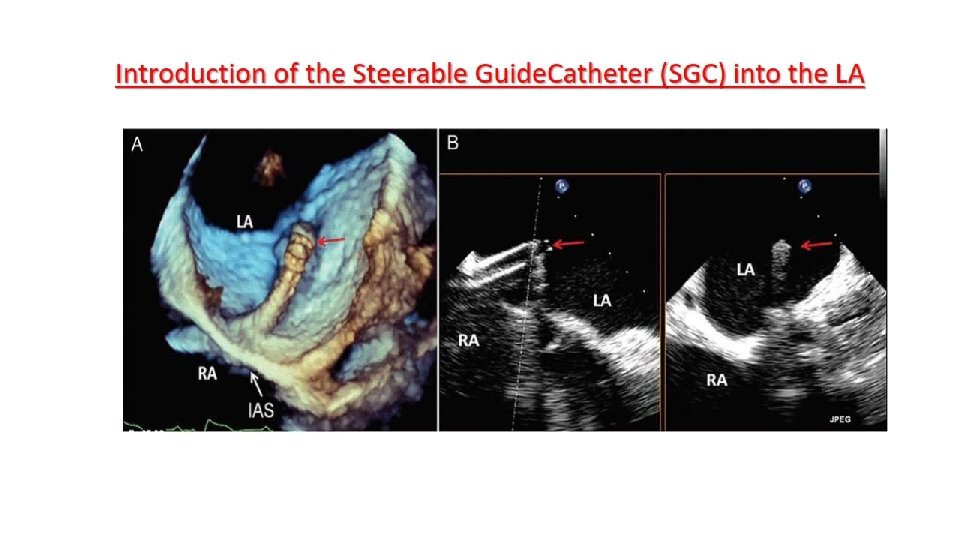
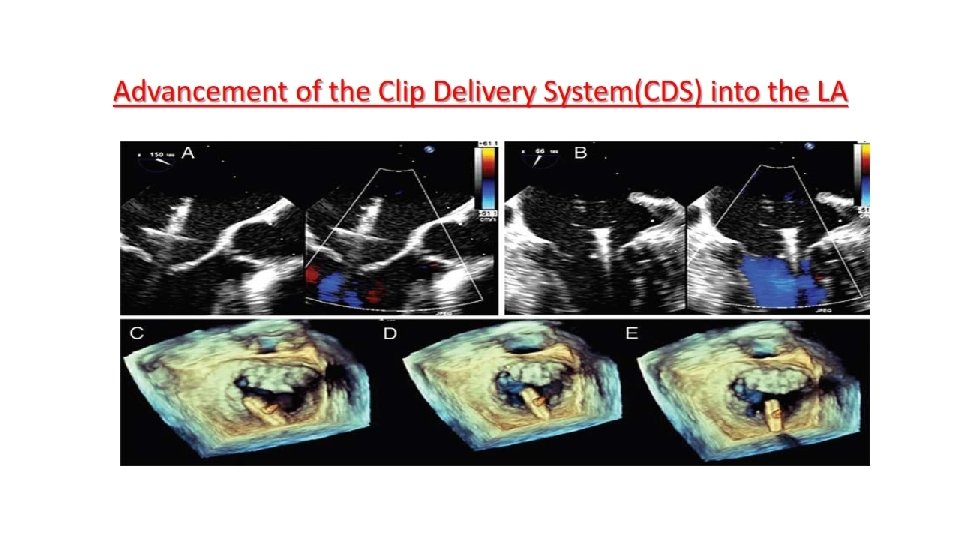
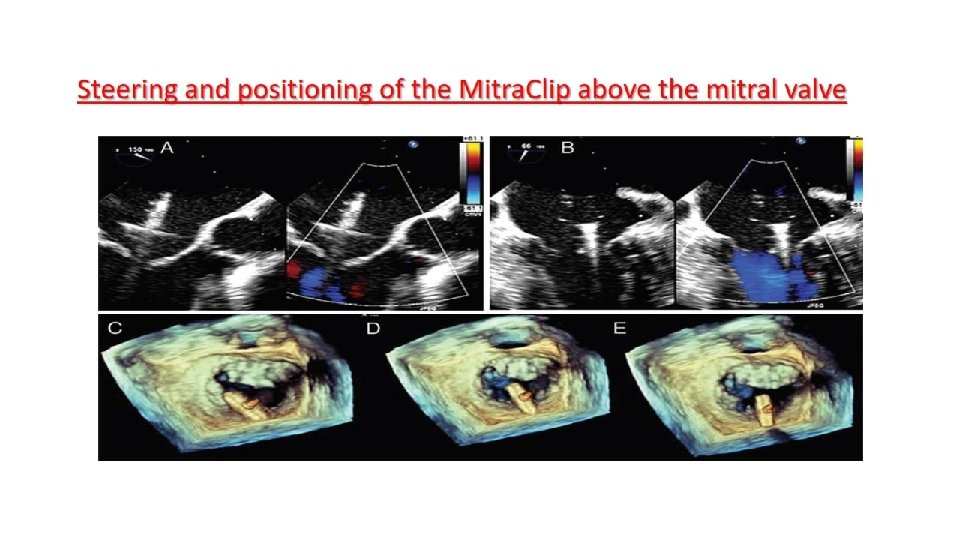
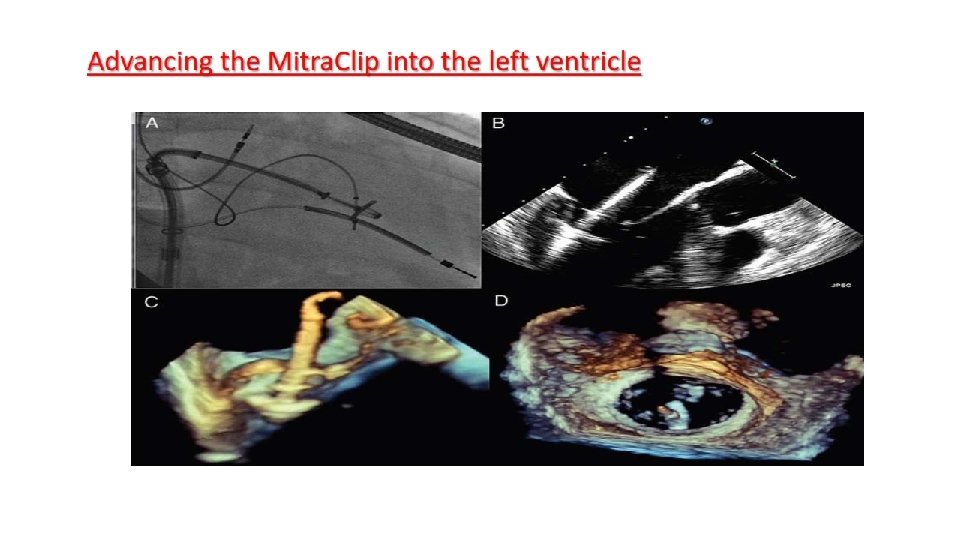
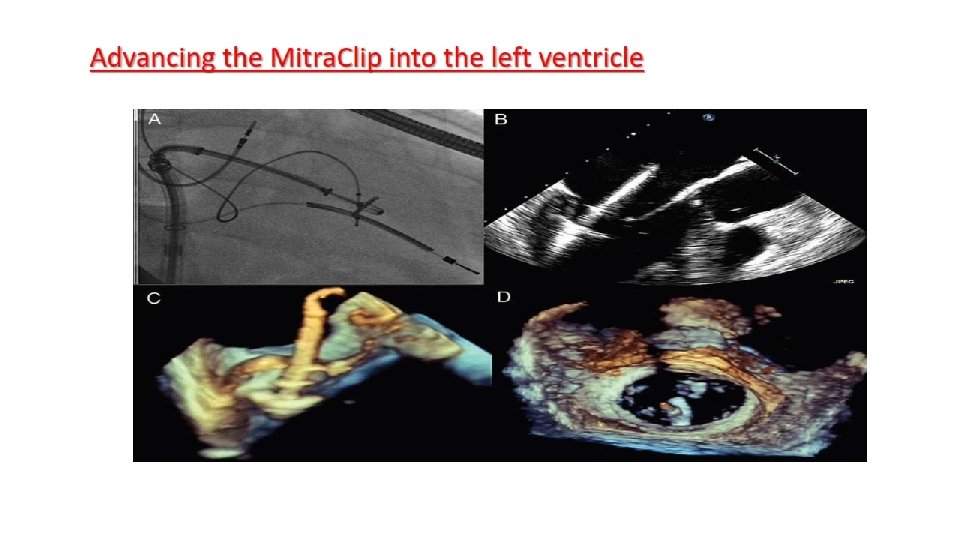
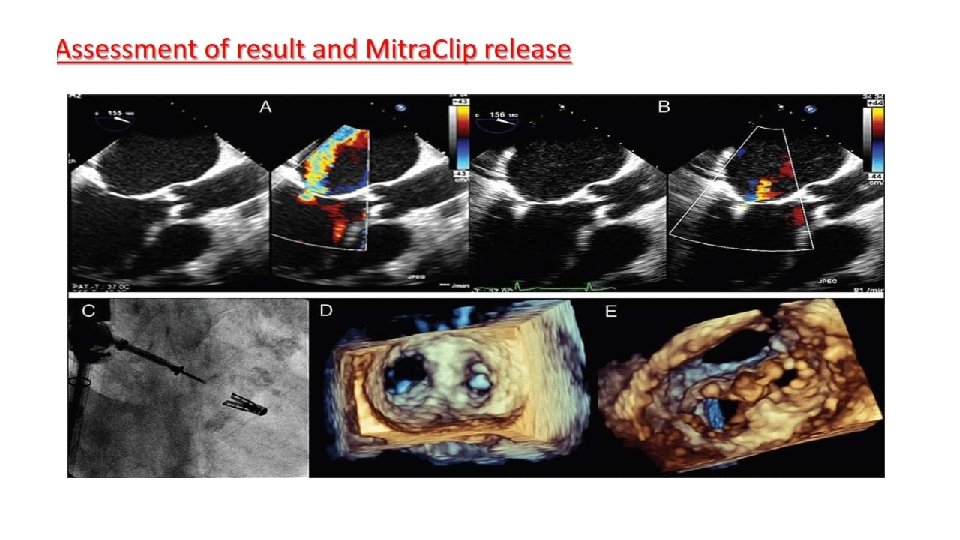
Main procedural steps for Mitra. Clip implantation











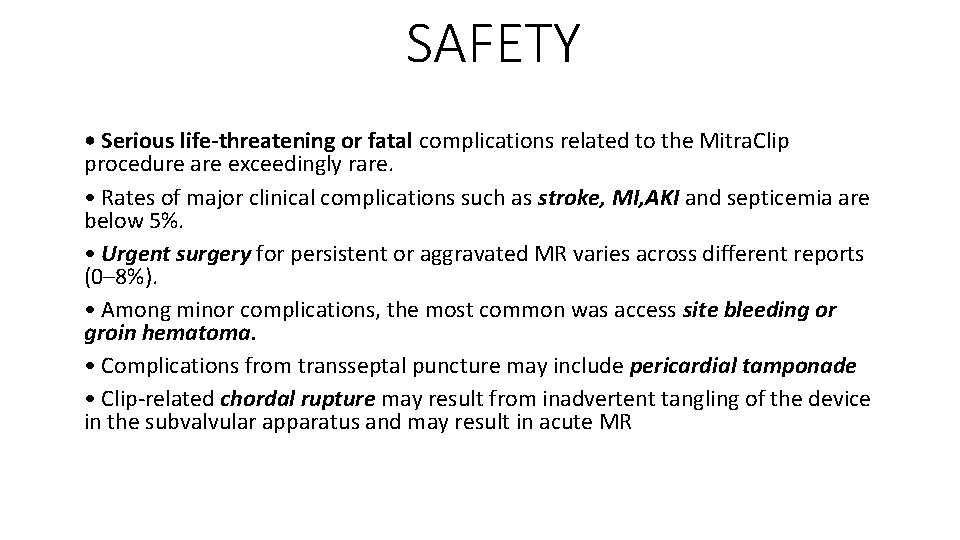
SAFETY • Serious life-threatening or fatal complications related to the Mitra. Clip procedure are exceedingly rare. • Rates of major clinical complications such as stroke, MI, AKI and septicemia are below 5%. • Urgent surgery for persistent or aggravated MR varies across different reports (0– 8%). • Among minor complications, the most common was access site bleeding or groin hematoma. • Complications from transseptal puncture may include pericardial tamponade • Clip-related chordal rupture may result from inadvertent tangling of the device in the subvalvular apparatus and may result in acute MR

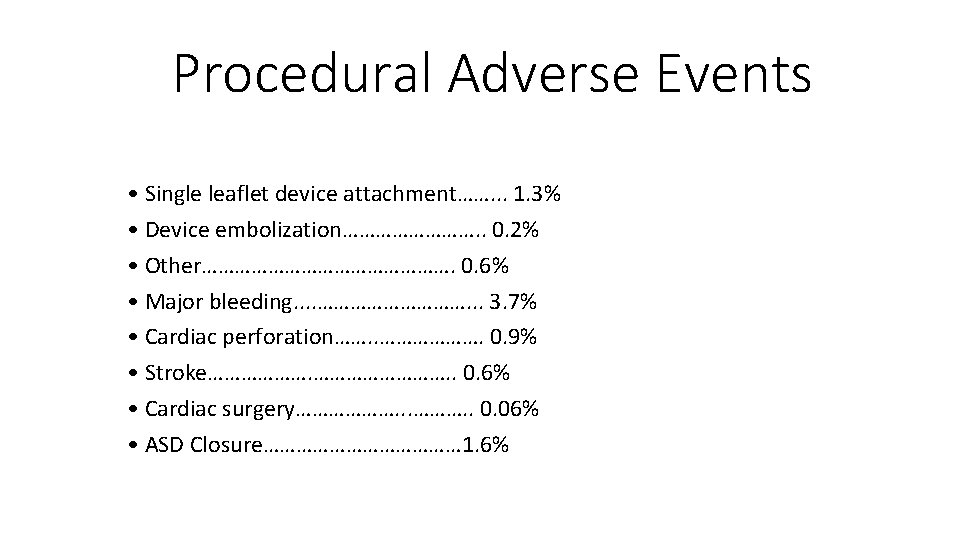
Procedural Adverse Events • Single leaflet device attachment……. . . 1. 3% • Device embolization…………. . 0. 2% • Other……………………. 0. 6% • Major bleeding. . ……………. . . 3. 7% • Cardiac perforation……. . ………………. 0. 9% • Stroke………………. . 0. 6% • Cardiac surgery………………. . 0. 06% • ASD Closure……………… 1. 6%

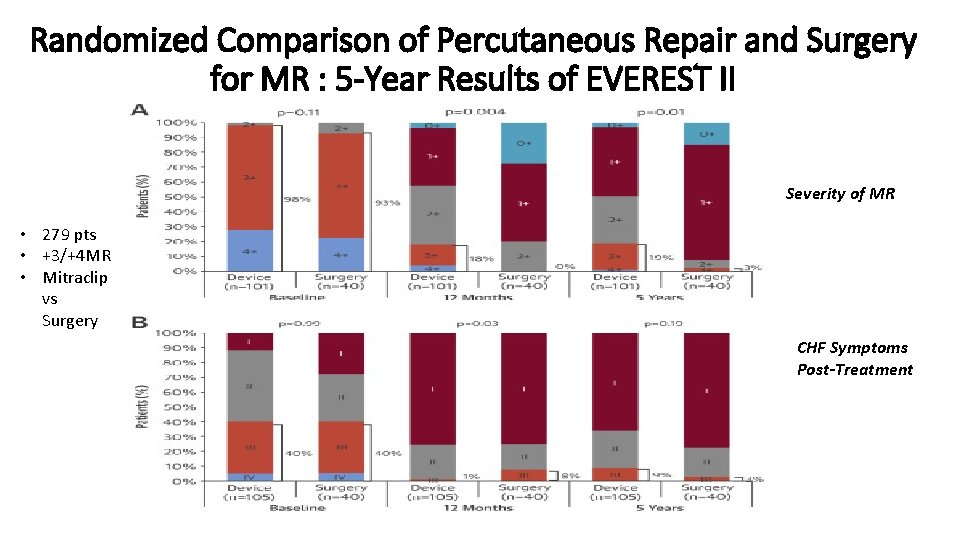
Randomized Comparison of Percutaneous Repair and Surgery for MR : 5 -Year Results of EVEREST II Severity of MR • 279 pts • +3/+4 MR • Mitraclip vs Surgery CHF Symptoms Post-Treatment

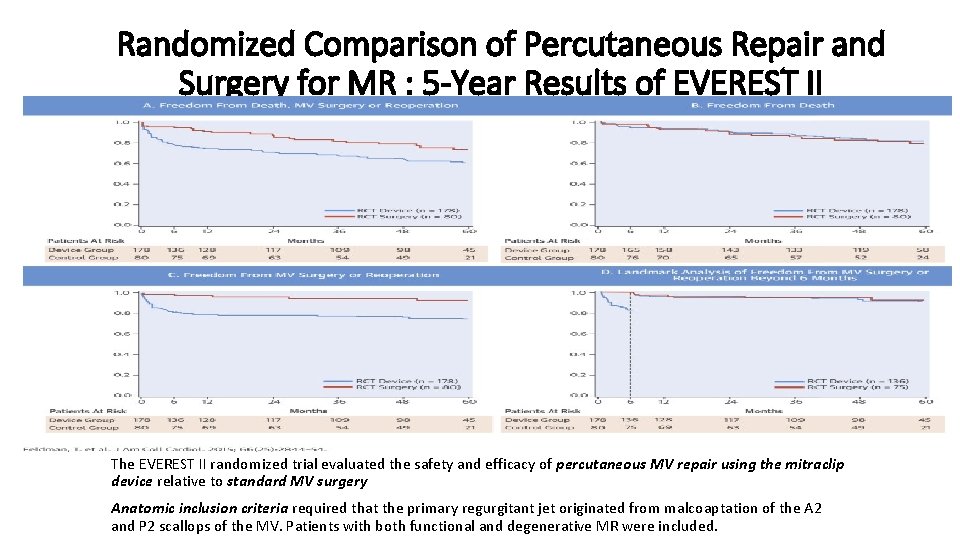
Randomized Comparison of Percutaneous Repair and Surgery for MR : 5 -Year Results of EVEREST II The EVEREST II randomized trial evaluated the safety and efficacy of percutaneous MV repair using the mitraclip device relative to standard MV surgery Anatomic inclusion criteria required that the primary regurgitant jet originated from malcoaptation of the A 2 and P 2 scallops of the MV. Patients with both functional and degenerative MR were included.

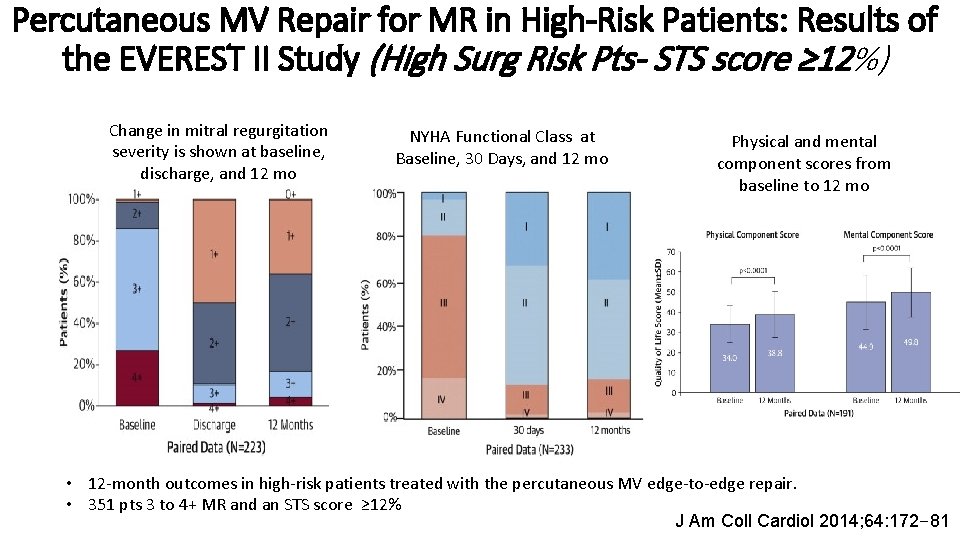
Percutaneous MV Repair for MR in High-Risk Patients: Results of the EVEREST II Study (High Surg Risk Pts- STS score ≥ 12%) Change in mitral regurgitation severity is shown at baseline, discharge, and 12 mo NYHA Functional Class at Baseline, 30 Days, and 12 mo Physical and mental component scores from baseline to 12 mo • 12 -month outcomes in high-risk patients treated with the percutaneous MV edge-to-edge repair. • 351 pts 3 to 4+ MR and an STS score ≥ 12% J Am Coll Cardiol 2014; 64: 172– 81

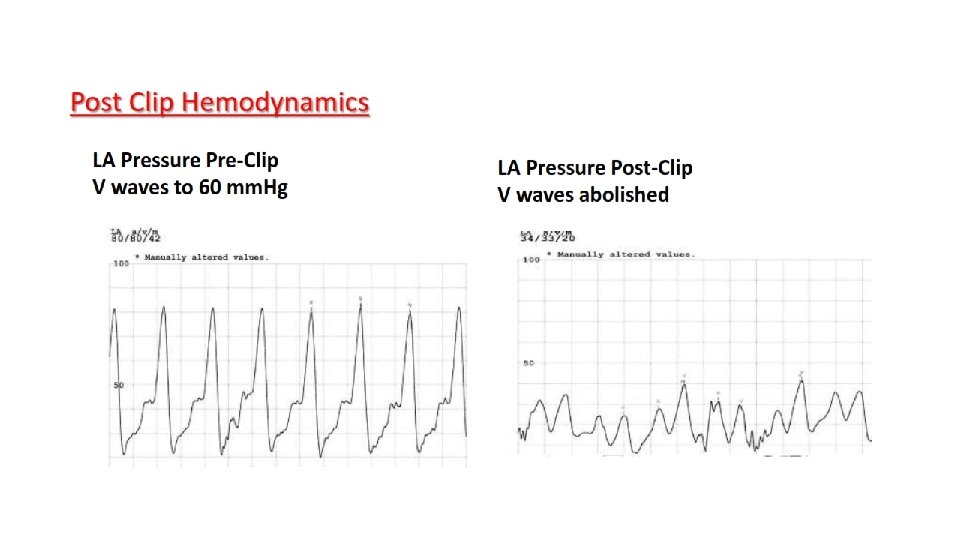
What We Learned from EVEREST II Five-Year Data Surgery vs Mitra. Clip in a Mixed DMR/FMR Population 1. At 1 year, percutaneous MV repair was less effective than surgery in reducing MR and there more re-operations in the Mitra. Clip arm 2. But there were no differences between surgery and Mitra. Clip in safety, relief of symptoms, QOL and survival. 3. Performing Mitra. Clip you need to achieve: a. Two grade reduction in MR severity. b. Amelioration of PV flow reversal. c. Mean transvalvular GR < 5. 0 mm. Hg.

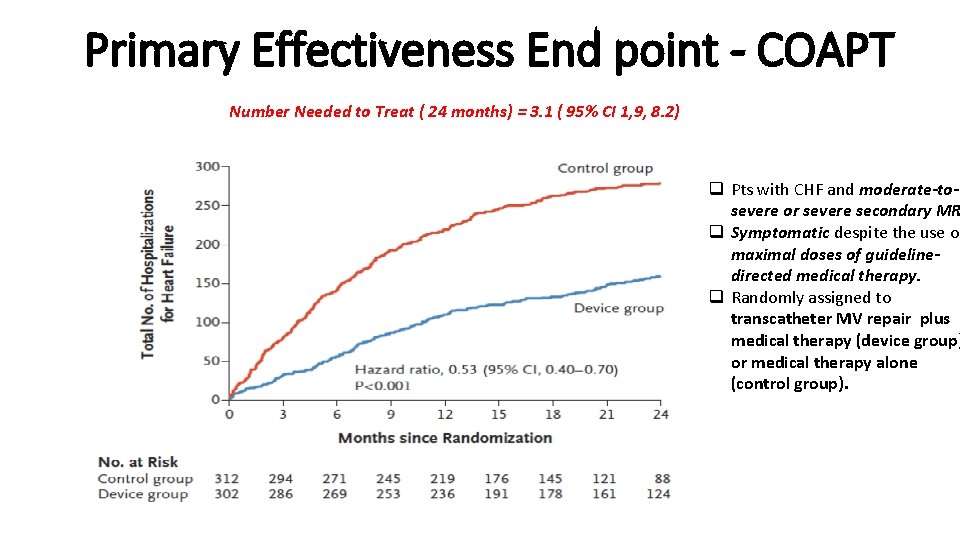
Primary Effectiveness End point - COAPT Number Needed to Treat ( 24 months) = 3. 1 ( 95% CI 1, 9, 8. 2) q Pts with CHF and moderate-tosevere or severe secondary MR q Symptomatic despite the use of maximal doses of guidelinedirected medical therapy. q Randomly assigned to transcatheter MV repair plus medical therapy (device group) or medical therapy alone (control group).

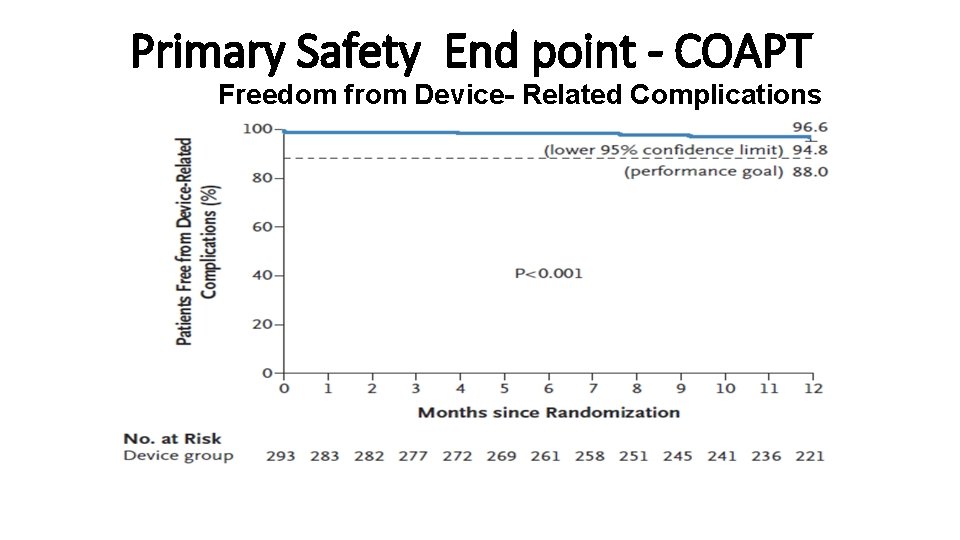
Primary Safety End point - COAPT Freedom from Device- Related Complications

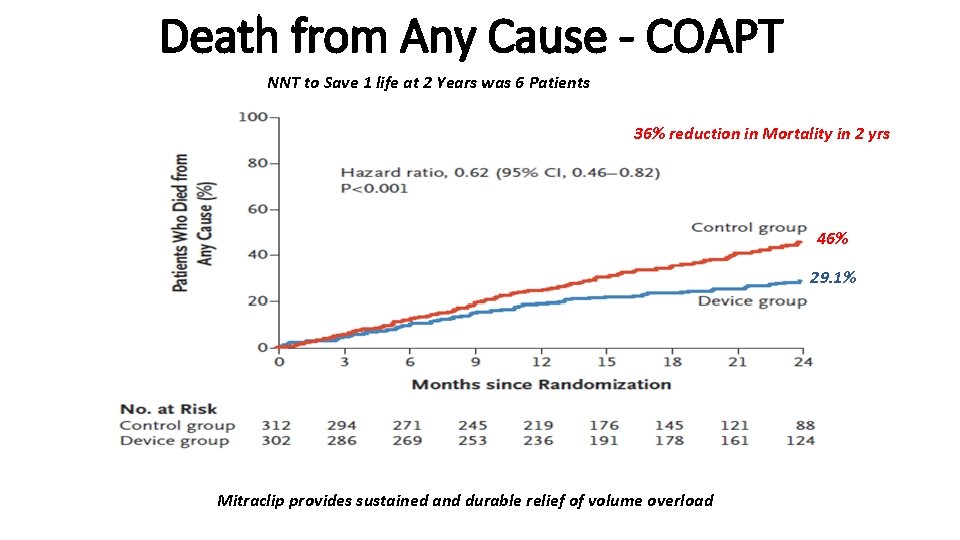
Death from Any Cause - COAPT NNT to Save 1 life at 2 Years was 6 Patients 36% reduction in Mortality in 2 yrs 46% 29. 1% Mitraclip provides sustained and durable relief of volume overload

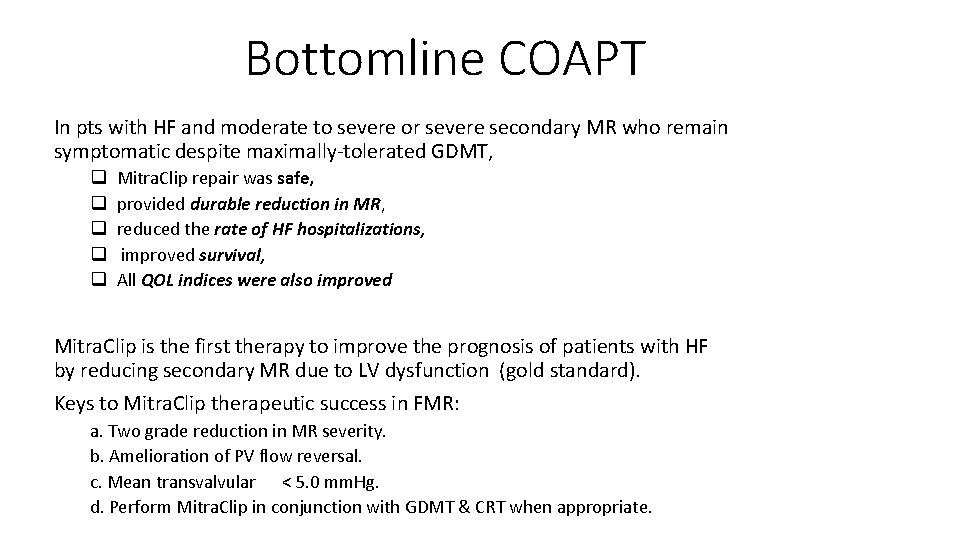
Bottomline COAPT In pts with HF and moderate to severe or severe secondary MR who remain symptomatic despite maximally-tolerated GDMT, q q q Mitra. Clip repair was safe, provided durable reduction in MR, reduced the rate of HF hospitalizations, improved survival, All QOL indices were also improved Mitra. Clip is the first therapy to improve the prognosis of patients with HF by reducing secondary MR due to LV dysfunction (gold standard). Keys to Mitra. Clip therapeutic success in FMR: a. Two grade reduction in MR severity. b. Amelioration of PV flow reversal. c. Mean transvalvular < 5. 0 mm. Hg. d. Perform Mitra. Clip in conjunction with GDMT & CRT when appropriate.

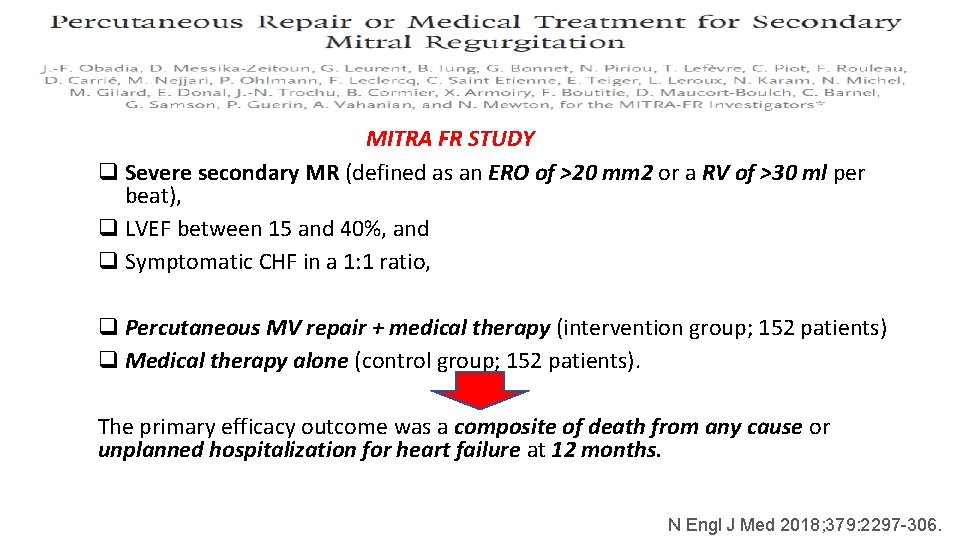
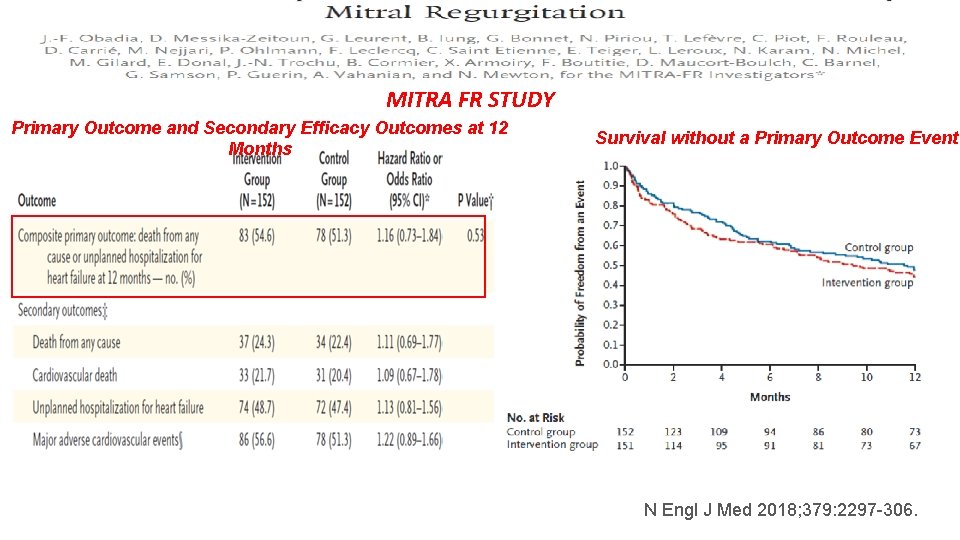
MITRA FR STUDY q Severe secondary MR (defined as an ERO of >20 mm 2 or a RV of >30 ml per beat), q LVEF between 15 and 40%, and q Symptomatic CHF in a 1: 1 ratio, q Percutaneous MV repair + medical therapy (intervention group; 152 patients) q Medical therapy alone (control group; 152 patients). The primary efficacy outcome was a composite of death from any cause or unplanned hospitalization for heart failure at 12 months. N Engl J Med 2018; 379: 2297 -306.

MITRA FR STUDY Primary Outcome and Secondary Efficacy Outcomes at 12 Months Survival without a Primary Outcome Event N Engl J Med 2018; 379: 2297 -306.

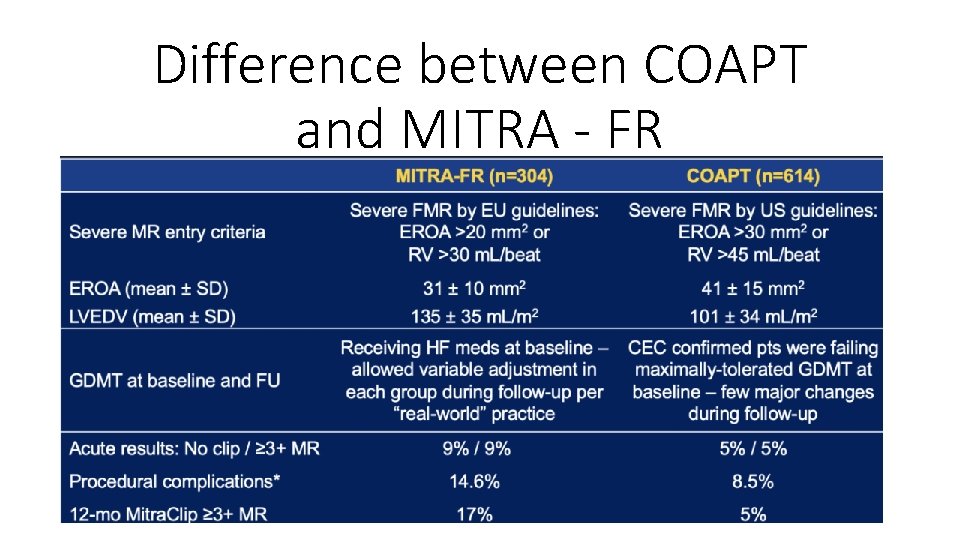
Difference between COAPT and MITRA - FR

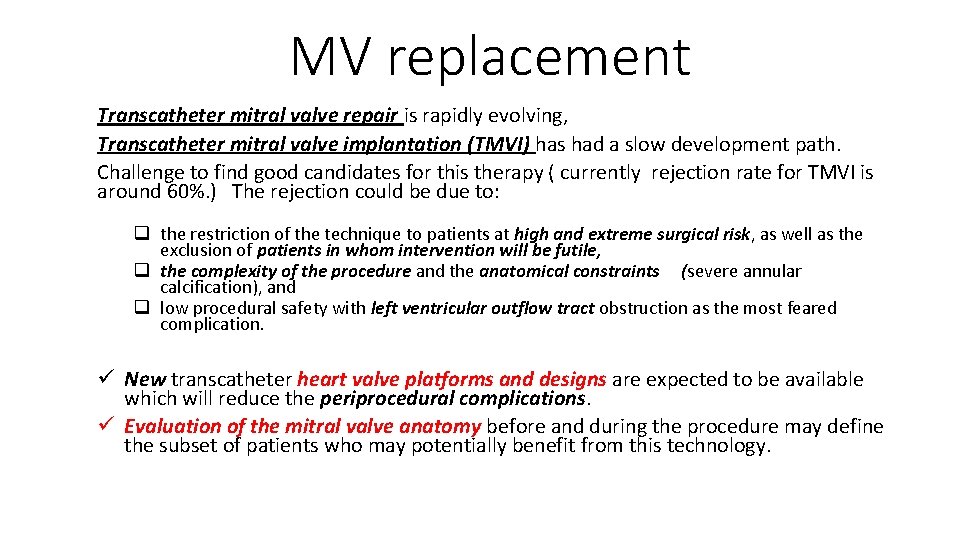
MV replacement Transcatheter mitral valve repair is rapidly evolving, Transcatheter mitral valve implantation (TMVI) has had a slow development path. Challenge to find good candidates for this therapy ( currently rejection rate for TMVI is around 60%. ) The rejection could be due to: q the restriction of the technique to patients at high and extreme surgical risk, as well as the exclusion of patients in whom intervention will be futile, q the complexity of the procedure and the anatomical constraints (severe annular calcification), and q low procedural safety with left ventricular outflow tract obstruction as the most feared complication. ü New transcatheter heart valve platforms and designs are expected to be available which will reduce the periprocedural complications. ü Evaluation of the mitral valve anatomy before and during the procedure may define the subset of patients who may potentially benefit from this technology.

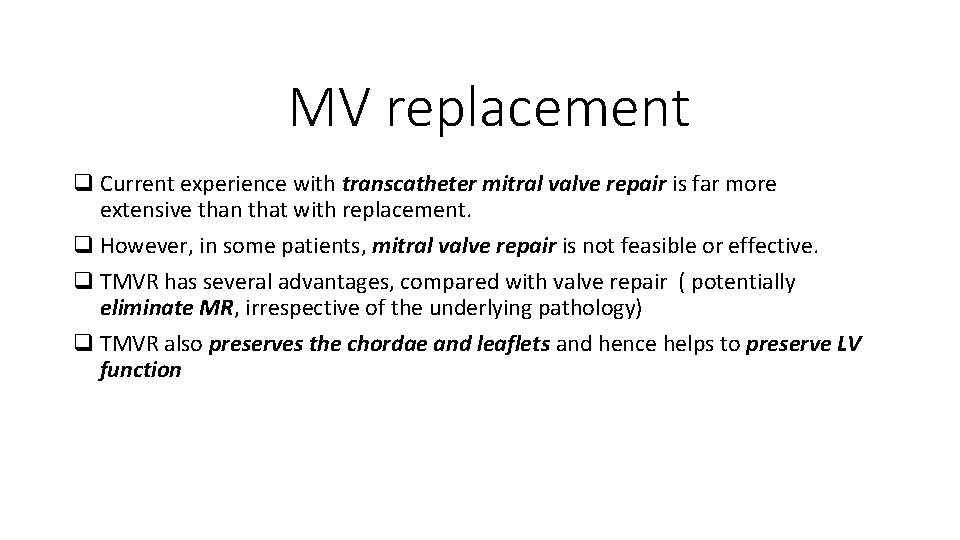
MV replacement q Current experience with transcatheter mitral valve repair is far more extensive than that with replacement. q However, in some patients, mitral valve repair is not feasible or effective. q TMVR has several advantages, compared with valve repair ( potentially eliminate MR, irrespective of the underlying pathology) q TMVR also preserves the chordae and leaflets and hence helps to preserve LV function

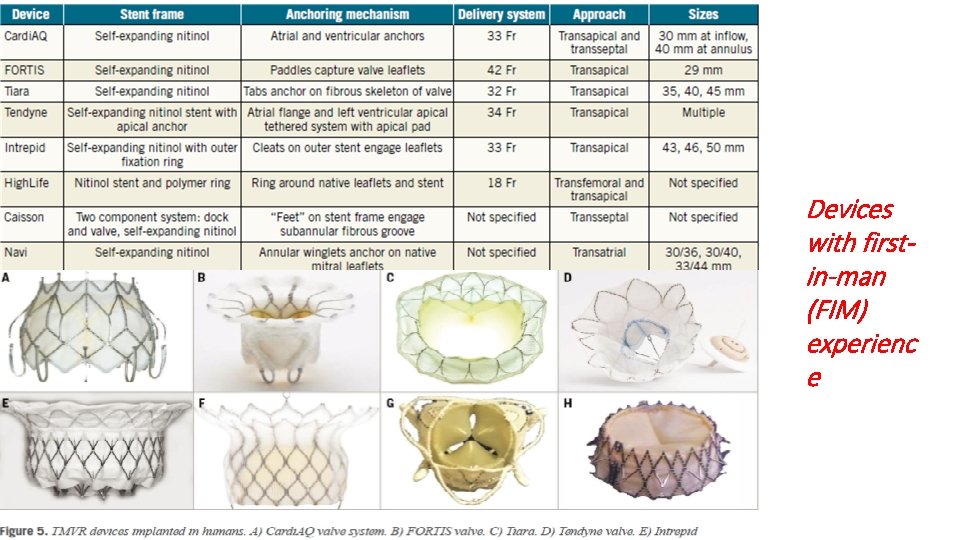
Devices with firstin-man (FIM) experienc e

Conclusions Ø The mitral valve is the next frontier in structural valve disease after the success of TAVI. Ø Data on long-term outcomes establish the safety, efficacy, and durability of transcatheter approaches to mitral valve repair and replacement. Ø Appropriate patient selection needed for optimal results. Ø Better understanding of the anatomical and physiological factors of each device and pathology Ø Preprocedural planning, plays a critical role in device selection and procedural guidance (multimodality CT and TEE) Ø The need for device specific anticoagulation will also be important. Anticoagulation strategy is still unclear (? ) Ø TA approach will probably remain the most common access site for TMVR , but developments in the delivery system and device profile will move this technology towards the less invasive TS approach.