Mitra Clip A Therapeutic Solution for Patients with












- Slides: 44

Mitra. Clip: A Therapeutic Solution for Patients with Severe MR – Not a Candidate for Surgery Brij Maini MD, FACC Regional Medical Director of Transcatheter Therapies Tenet Healthcare Corporation Florida Region

Disclosure Statement of Financial Interest Brijeshwar Maini MD, FACC Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Grant/Research Support • Consulting Fees/Honoraria Company • • • Abbott Vascular Medtronic Abiomed SJM Siemens Atritech/Boston Scientific

Background • Degenerative MR is common, affecting ~600, 000 persons in the U. S. • Surgery is the standard of care, and is indicated for patients with symptoms or LV dysfunction • However, there are patients in whom the risk of surgery is prohibitive

• EVEREST • TVT • OUR EXPERIENCE

Methods Determination of Prohibitive Risk • 141 high surgical risk DMR patients treated in the EVEREST Registries with 1 -year follow-up available • Prohibitive risk definition retrospectively applied by heart team − Two cardiac surgeons experienced in MV surgery − Cardiologist experienced in MV disease • 127 patients were documented to meet prohibitive risk

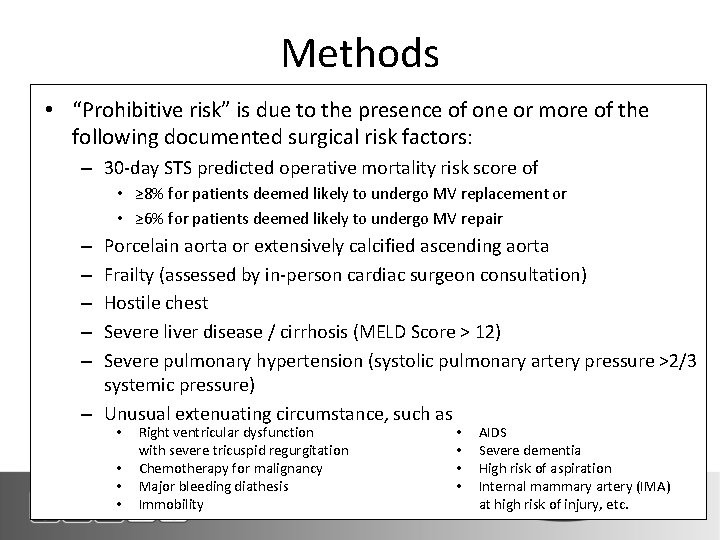
Methods Definition of Prohibitive Risk • “Prohibitive risk” is due to the presence of one or more of the following documented surgical risk factors: – 30 -day STS predicted operative mortality risk score of • ≥ 8% for patients deemed likely to undergo MV replacement or • ≥ 6% for patients deemed likely to undergo MV repair Porcelain aorta or extensively calcified ascending aorta Frailty (assessed by in-person cardiac surgeon consultation) Hostile chest Severe liver disease / cirrhosis (MELD Score > 12) Severe pulmonary hypertension (systolic pulmonary artery pressure >2/3 systemic pressure) – Unusual extenuating circumstance, such as – – – • • Right ventricular dysfunction with severe tricuspid regurgitation Chemotherapy for malignancy Major bleeding diathesis Immobility • • AIDS Severe dementia High risk of aspiration Internal mammary artery (IMA) at high risk of injury, etc.

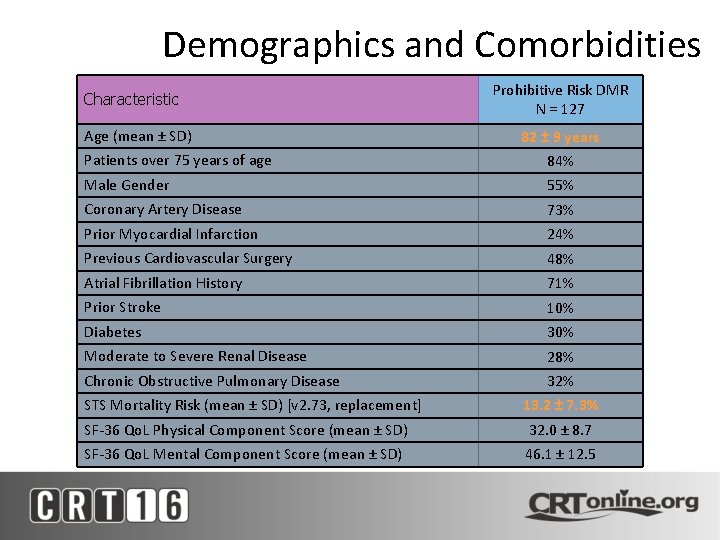
Baseline Demographics and Comorbidities Characteristic Age (mean ± SD) Prohibitive Risk DMR N = 127 82 ± 9 years Patients over 75 years of age 84% Male Gender 55% Coronary Artery Disease 73% Prior Myocardial Infarction 24% Previous Cardiovascular Surgery 48% Atrial Fibrillation History 71% Prior Stroke 10% Diabetes 30% Moderate to Severe Renal Disease 28% Chronic Obstructive Pulmonary Disease STS Mortality Risk (mean ± SD) [v 2. 73, replacement] 32% 13. 2 ± 7. 3% SF-36 Qo. L Physical Component Score (mean ± SD) 32. 0 ± 8. 7 SF-36 Qo. L Mental Component Score (mean ± SD) 46. 1 ± 12. 5

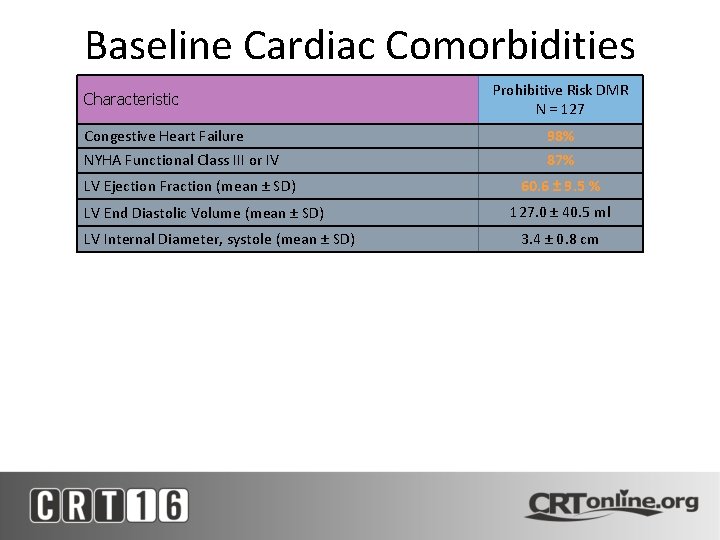
Baseline Cardiac Comorbidities Characteristic Prohibitive Risk DMR N = 127 Congestive Heart Failure 98% NYHA Functional Class III or IV 87% LV Ejection Fraction (mean ± SD) LV End Diastolic Volume (mean ± SD) LV Internal Diameter, systole (mean ± SD) 60. 6 ± 9. 5 % 127. 0 ± 40. 5 ml 3. 4 ± 0. 8 cm

Reasons For Prohibitive Surgical Risk Prohibitive Risk Factor ╪ STS mortality risk score ≥ 8% (mean 13. 2 ± 7. 3%) Risk factors in patients with STS mortality risk score < 8% that are not captured in STS calculator: Porcelain Aorta Hostile chest Severe liver disease or cirrhosis Severe pulmonary hypertension Frailty Unusual extenuating circumstance: High risk of aspiration IMA at high risk of injury Major bleeding diathesis Severe dementia Chemotherapy for malignancy Immobility AIDS ╪Non-hierarchical Prohibitive Risk DMR N = 127 n % 101 79. 5% 8 5 4 3 2 6. 3% 3. 9% 3. 1% 2. 4% 1. 6% 4 4 2 2 1 1 1 3. 1% 1. 6% 0. 8% listing; 78 (61. 4%) patients presented with more than 1 prohibitive risk factor

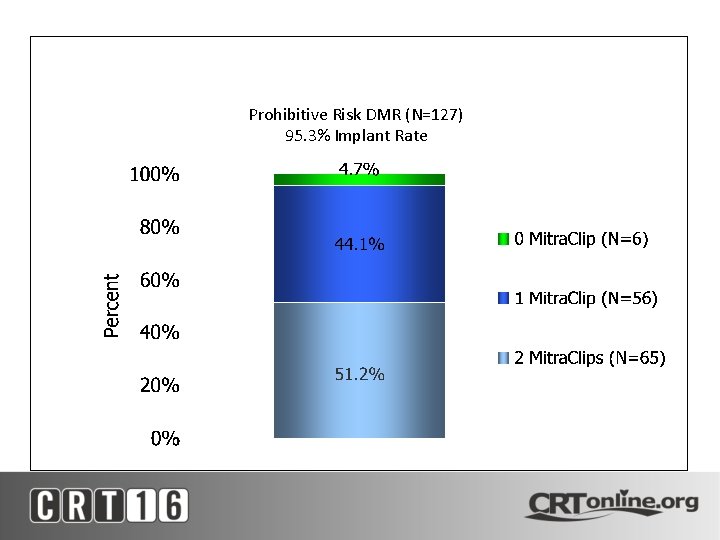
Mitra. Clip Implant Rate Prohibitive Risk DMR (N=127) 95. 3% Implant Rate

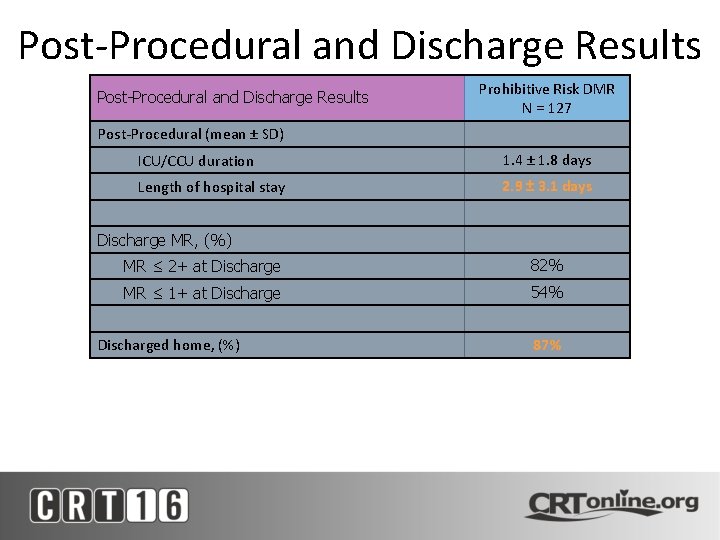
Post-Procedural and Discharge Results Prohibitive Risk DMR N = 127 Post-Procedural (mean ± SD) ICU/CCU duration 1. 4 ± 1. 8 days Length of hospital stay 2. 9 ± 3. 1 days Discharge MR, (%) MR ≤ 2+ at Discharge 82% MR ≤ 1+ at Discharge 54% Discharged home, (%) 87%

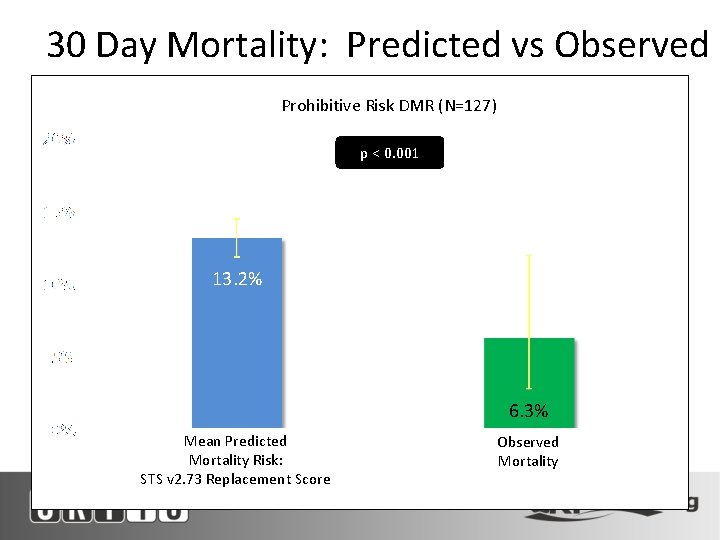
30 Day Mortality: Predicted vs Observed Prohibitive Risk DMR (N=127) p < 0. 001 13. 2% 6. 3% Mean Predicted Mortality Risk: STS v 2. 73 Replacement Score Observed Mortality

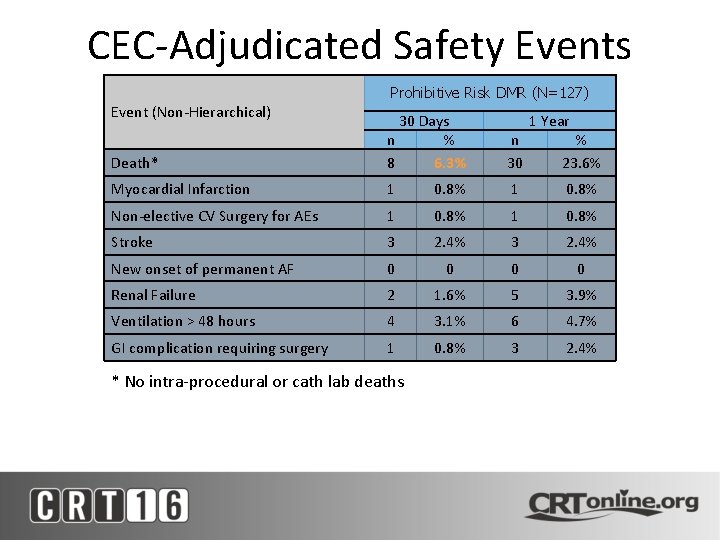
CEC-Adjudicated Safety Events Prohibitive Risk DMR (N=127) Event (Non-Hierarchical) Death* 30 Days n % 8 6. 3% n 30 Myocardial Infarction 1 0. 8% Non-elective CV Surgery for AEs 1 0. 8% Stroke 3 2. 4% New onset of permanent AF 0 0 Renal Failure 2 1. 6% 5 3. 9% Ventilation > 48 hours 4 3. 1% 6 4. 7% GI complication requiring surgery 1 0. 8% 3 2. 4% * No intra-procedural or cath lab deaths 1 Year % 23. 6%

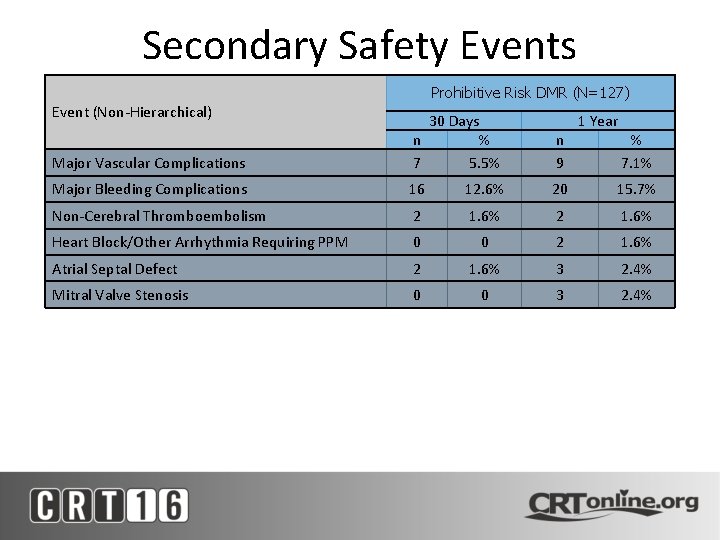
Secondary Safety Events Prohibitive Risk DMR (N=127) Event (Non-Hierarchical) Major Vascular Complications 30 Days n % 7 5. 5% 1 Year n 9 Major Bleeding Complications 16 12. 6% 20 15. 7% Non-Cerebral Thromboembolism 2 1. 6% Heart Block/Other Arrhythmia Requiring PPM 0 0 2 1. 6% Atrial Septal Defect 2 1. 6% 3 2. 4% Mitral Valve Stenosis 0 0 3 2. 4% % 7. 1%

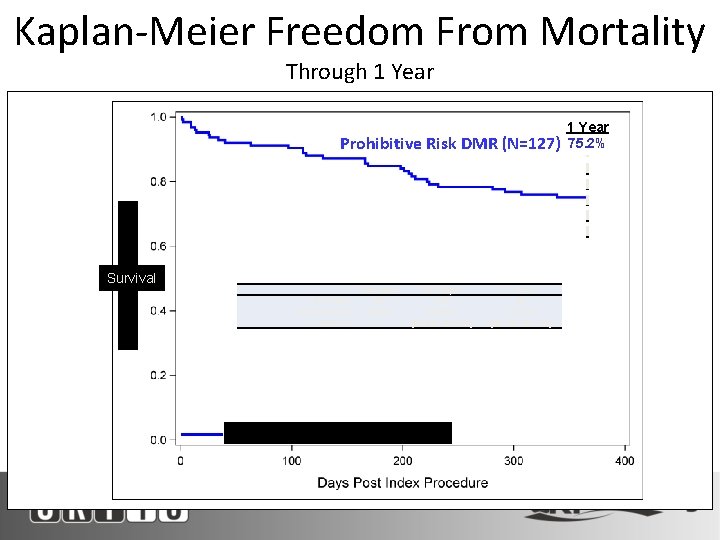
Kaplan-Meier Freedom From Mortality Through 1 Year Prohibitive Risk DMR (N=127) Survival # At Risk % Event Free 95% CI Baseline 127 100% - 30 Days 117 93. 6% [87. 6%, 96. 8%] Prohibitive Risk DMR Cohort (N=127) 1 Year 85 75. 2% [66. 1%, 82. 1%] 1 Year 75. 2%

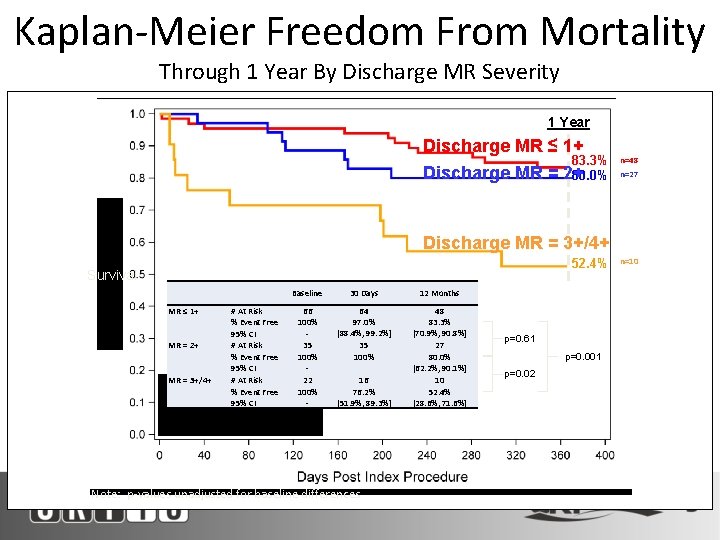
Kaplan-Meier Freedom From Mortality Through 1 Year By Discharge MR Severity 1 Year Discharge MR ≤ 1+ Discharge MR = 83. 3% 2+ 80. 0% n=48 n=27 Discharge MR = 3+/4+ 52. 4% Survival MR ≤ 1+ MR = 2+ MR = 3+/4+ # At Risk % Event Free 95% CI Baseline 30 Days 12 Months 66 100% 35 100% 22 100% - 64 97. 0% [88. 4%, 99. 2%] 35 100% 16 76. 2% [51. 9%, 89. 3%] 48 83. 3% [70. 9%, 90. 8%] 27 80. 0% [62. 2%, 90. 1%] 10 52. 4% [28. 6%, 71. 6%] Note: p-values unadjusted for baseline differences p=0. 61 p=0. 001 p=0. 02 n=10

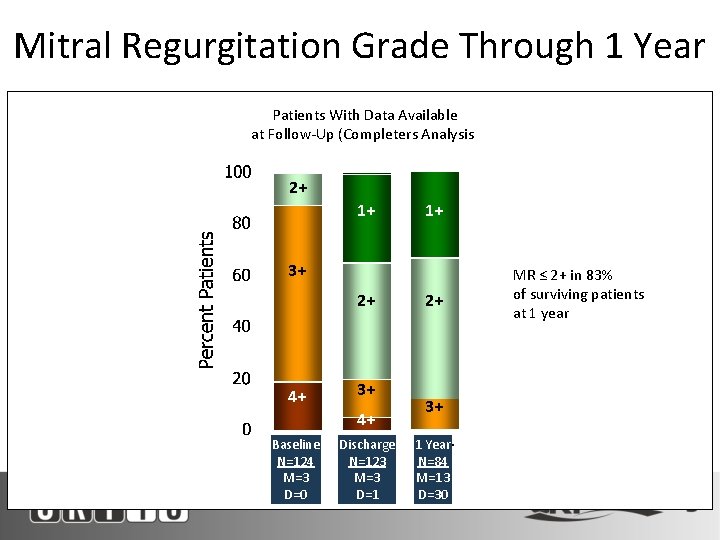
Mitral Regurgitation Grade Through 1 Year Patients With Data Available at Follow-Up (Completers Analysis) 2+ 1+ 1+ 3+ 2+ 4+ 3+ 4+ M=missing, D=died Baseline N=124 M=3 D=0 Discharge N=123 M=3 D=1 2+ 3+ 1 Year N=84 M=13 D=30 MR ≤ 2+ in 83% of surviving patients at 1 year

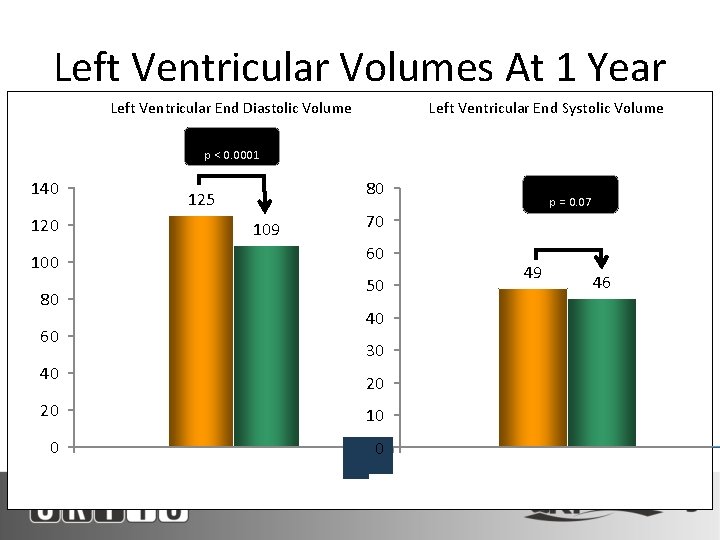
Left Ventricular Volumes At 1 Year Left Ventricular End Diastolic Volume Left Ventricular End Systolic Volume ∆ = -16 m. L p < 0. 0001 140 80 125 120 109 50 80 46 30 40 20 20 N Denotes Paired Survivors 49 40 60 0 70 60 100 ∆ = -3 m. L p = 0. 07 10 Baseline N=69 1 Year N=69

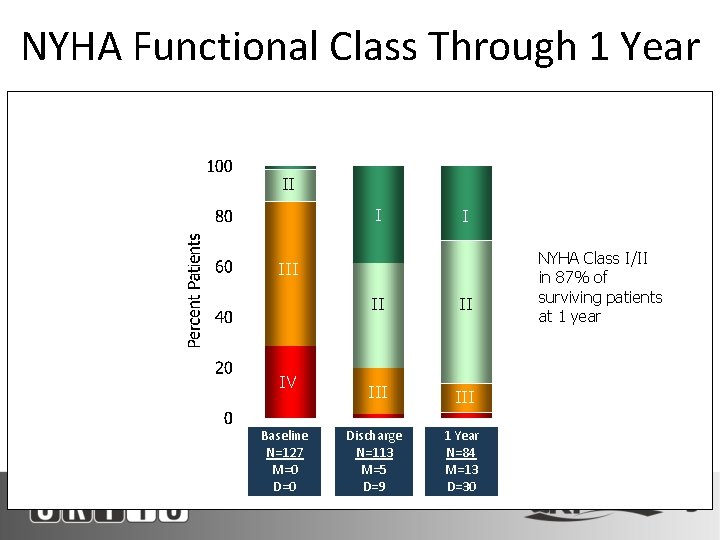
NYHA Functional Class Through 1 Year Patients With Data Available at Follow-Up (Completers Analysis) II 2+ I III IV M=missing, D=died Baseline N=127 M=0 D=0 I 3+ II II III 4+ III Discharge N=113 M=5 D=9 1 Year N=84 M=13 D=30 NYHA Class I/II in 87% of surviving patients at 1 year

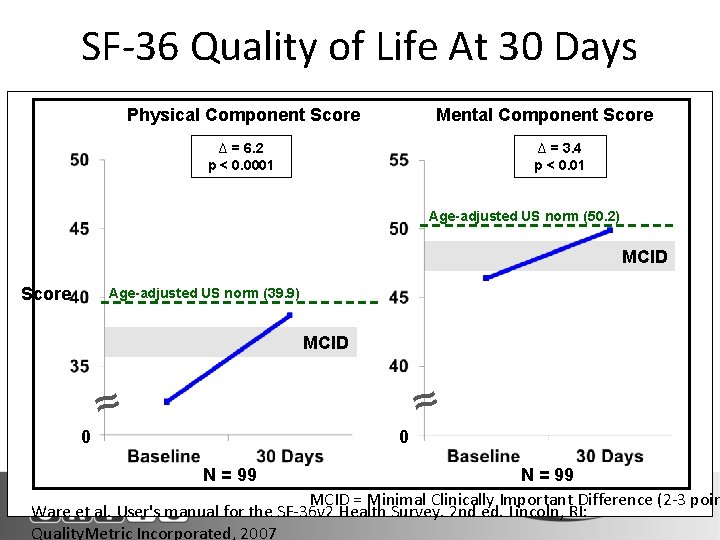
SF-36 Quality of Life At 30 Days Physical Component Score Mental Component Score ∆ = 6. 2 p < 0. 0001 ∆ = 3. 4 p < 0. 01 Age-adjusted US norm (50. 2) MCID Score Age-adjusted US norm (39. 9) MCID ≈ 0 0 ≈ N = 99 N Denotes Paired Survivors MCID = Minimal Clinically Important Difference (2 -3 poin Ware et al. User's manual for the SF-36 v 2 Health Survey. 2 nd ed. Lincoln, RI: Quality. Metric Incorporated, 2007

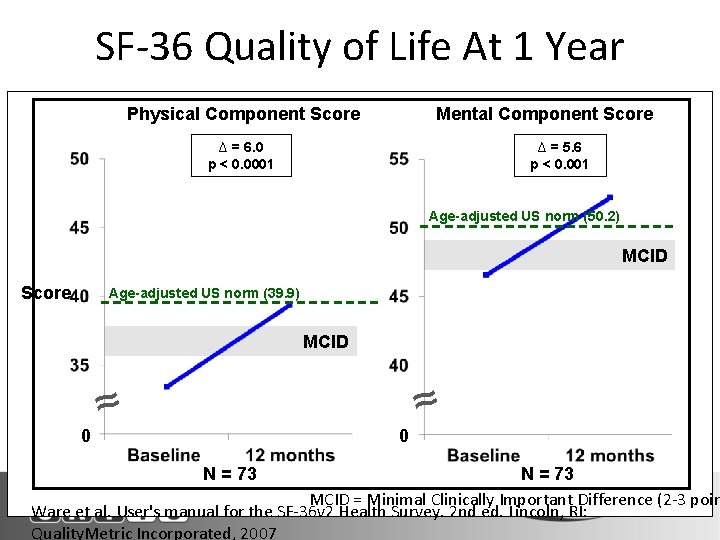
SF-36 Quality of Life At 1 Year Physical Component Score Mental Component Score ∆ = 6. 0 p < 0. 0001 ∆ = 5. 6 p < 0. 001 Age-adjusted US norm (50. 2) MCID Score Age-adjusted US norm (39. 9) MCID ≈ 0 0 ≈ N = 73 N Denotes Paired Survivors MCID = Minimal Clinically Important Difference (2 -3 poin Ware et al. User's manual for the SF-36 v 2 Health Survey. 2 nd ed. Lincoln, RI: Quality. Metric Incorporated, 2007

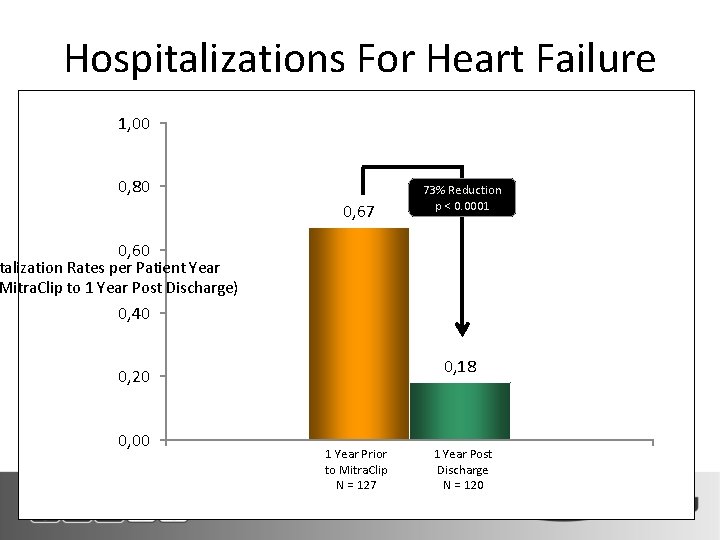
Hospitalizations For Heart Failure 1, 00 0, 80 0, 67 73% Reduction p < 0. 0001 0, 60 talization Rates per Patient Year Mitra. Clip to 1 Year Post Discharge) 0, 40 0, 18 0, 20 0, 00 1 Year Prior to Mitra. Clip N = 127 1 Year Post Discharge N = 120

Summary § Mitra. Clip therapy safely reduces DMR in patients at prohibitive risk for MV surgery § In this group of prohibitive risk DMR patients, Mitra. Clip therapy provides meaningful clinical improvements § § Reduction of LV volumes Improvements in NYHA Functional Class Improvements in Quality of Life Reduction in Hospitalizations for Heart Failure

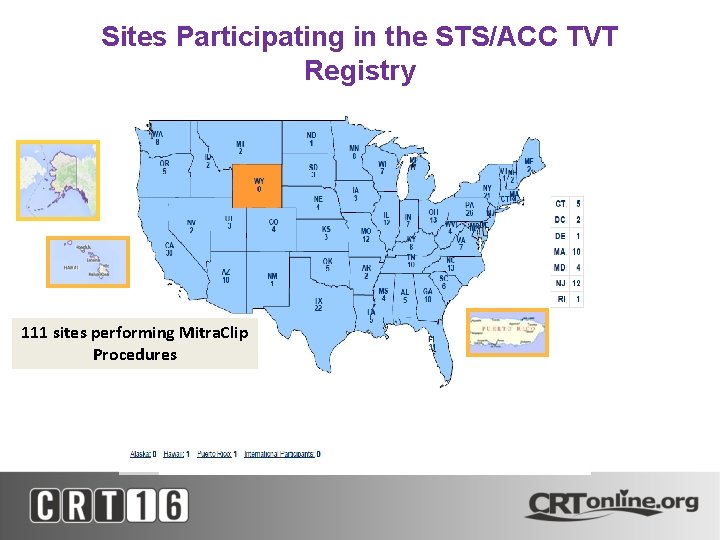
Sites Participating in the STS/ACC TVT Registry 111 sites performing Mitra. Clip Procedures

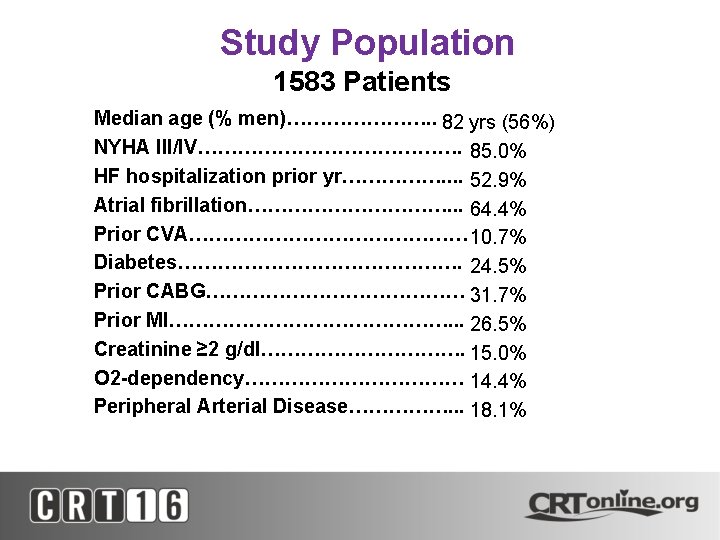
Study Population 1583 Patients • • • Median age (% men)…………………. . 82 yrs (56%) NYHA III/IV…………………. 85. 0% HF hospitalization prior yr……………. . 52. 9% Atrial fibrillation……………. . . 64. 4% Prior CVA………………… 10. 7% Diabetes…………………. 24. 5% Prior CABG………………… 31. 7% Prior MI…………………. . . 26. 5% Creatinine ≥ 2 g/dl……………. 15. 0% O 2 -dependency……………… 14. 4% Peripheral Arterial Disease……………. . . 18. 1%

Descriptors of Prohibitive Risk • Frailty……………. . . 50. 0% • Hostile chest…………. . . . . 7. 4% • Porcelain aorta…………………. . 2. 2% • RV dysfunction with severe TR…. …. . … 7. 8% • Immobility………………. . …… 8. 7% • Severe liver disease (MELD >12)……. . 1. 4% • IMA at high risk of injury…………………. . …. 4. 6% • Unusual extenuating circumstance………. … 28. 7% • Chemotherapy ………………. . 4. 6%

Surgical Risk for Mitral Repair By Site Median STS Score = 6. 2 [3. 9, 10]

Surgical Risk for Mitral Replacement By Site Median STS Score = 9. 5 [6, 14. 5]

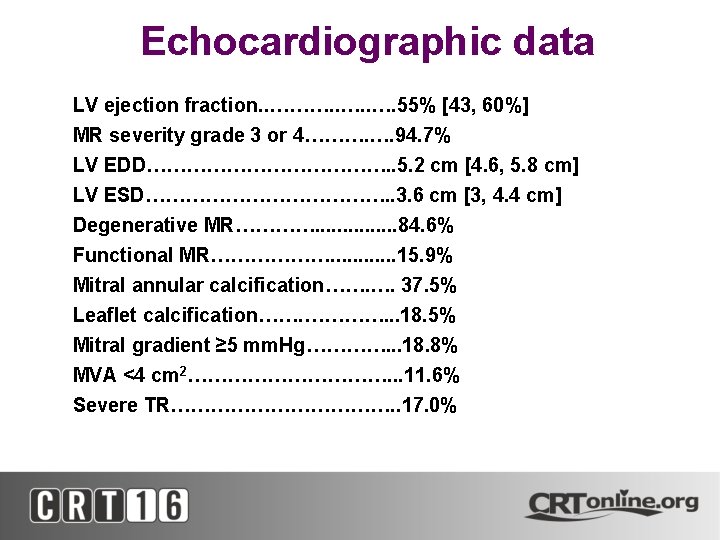
Echocardiographic data • • • LV ejection fraction. . ………. . …. 55% [43, 60%] MR severity grade 3 or 4………. …. 94. 7% LV EDD………………. . 5. 2 cm [4. 6, 5. 8 cm] LV ESD………………. . 3. 6 cm [3, 4. 4 cm] Degenerative MR…………. . . . 84. 6% Functional MR………………. . . 15. 9% Mitral annular calcification……. …. 37. 5% Leaflet calcification……. …………. . . 18. 5% Mitral gradient ≥ 5 mm. Hg…………. . . 18. 8% MVA <4 cm 2……………. . . 11. 6% Severe TR………………. . 17. 0%

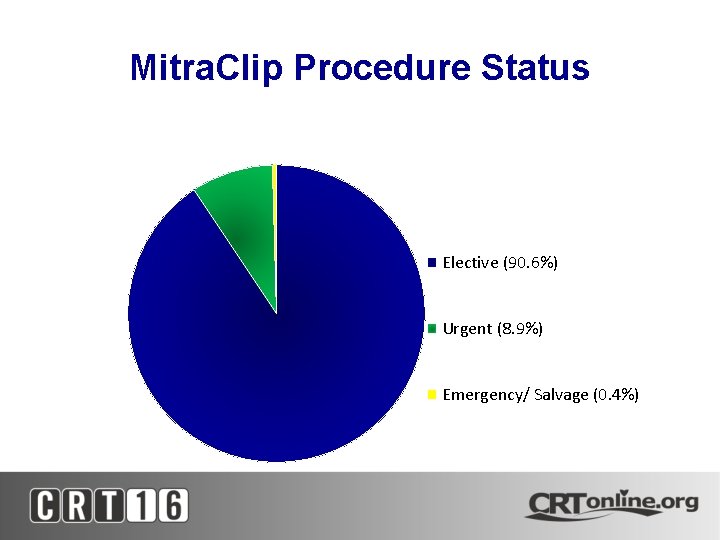
Mitra. Clip Procedure Status Elective (90. 6%) Urgent (8. 9%) Emergency/ Salvage (0. 4%)

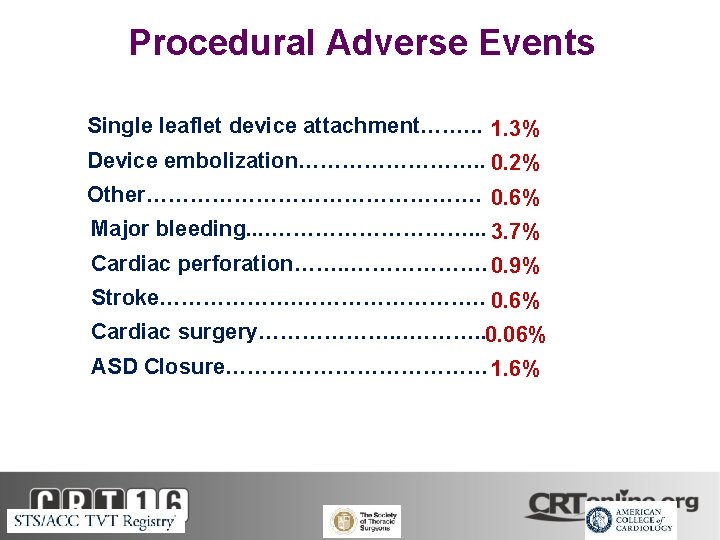
Procedural Adverse Events Single leaflet device attachment……. . . 1. 3% Device embolization…………. . 0. 2% Other……………………. 0. 6% Major bleeding. . ……………. . . 3. 7% Cardiac perforation……. . ………………. 0. 9% Stroke………………. . 0. 6% Cardiac surgery………………. . 0. 06% ASD Closure……………… 1. 6%

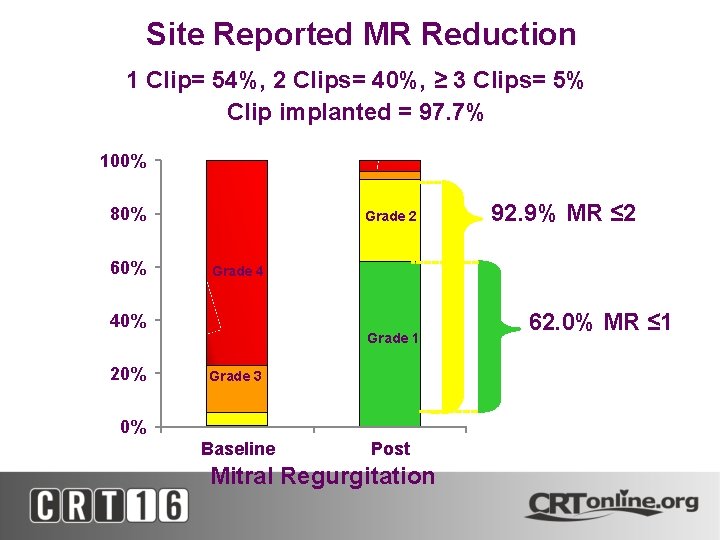
Site Reported MR Reduction 1 Clip= 54%, 2 Clips= 40%, ≥ 3 Clips= 5% Clip implanted = 97. 7% 100% 80% 60% Grade 2 Grade 4 40% 20% 92. 9% MR ≤ 2 Grade 1 Grade 3 0% Baseline Post Mitral Regurgitation 62. 0% MR ≤ 1

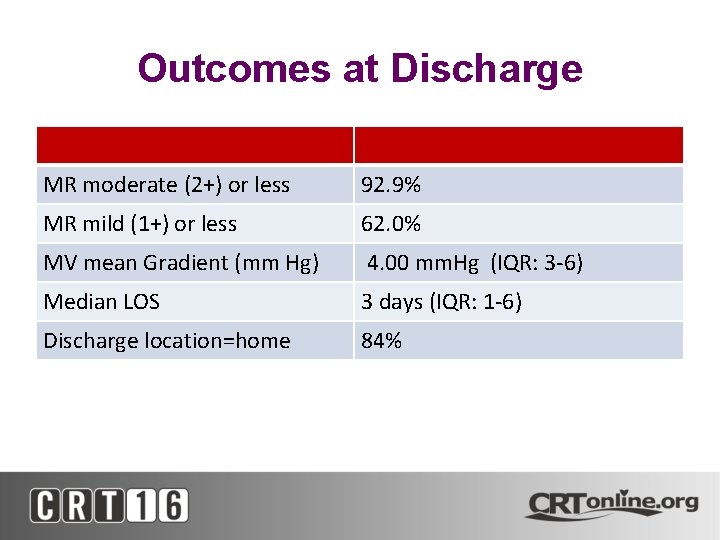
Outcomes at Discharge MR moderate (2+) or less 92. 9% MR mild (1+) or less 62. 0% MV mean Gradient (mm Hg) 4. 00 mm. Hg (IQR: 3 -6) Median LOS 3 days (IQR: 1 -6) Discharge location=home 84%

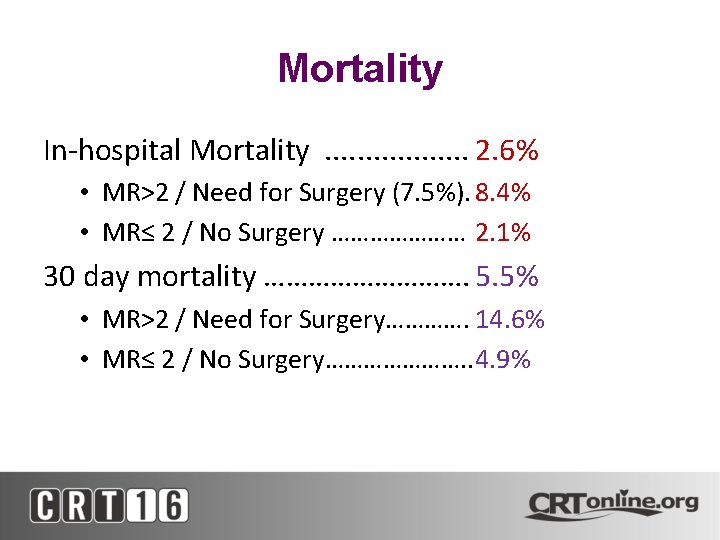
Mortality In-hospital Mortality. . . . 2. 6% • MR>2 / Need for Surgery (7. 5%). 8. 4% • MR≤ 2 / No Surgery ………………… 2. 1% 30 day mortality ……………. 5. 5% • MR>2 / Need for Surgery…………. 14. 6% • MR≤ 2 / No Surgery…………………. . 4. 9%

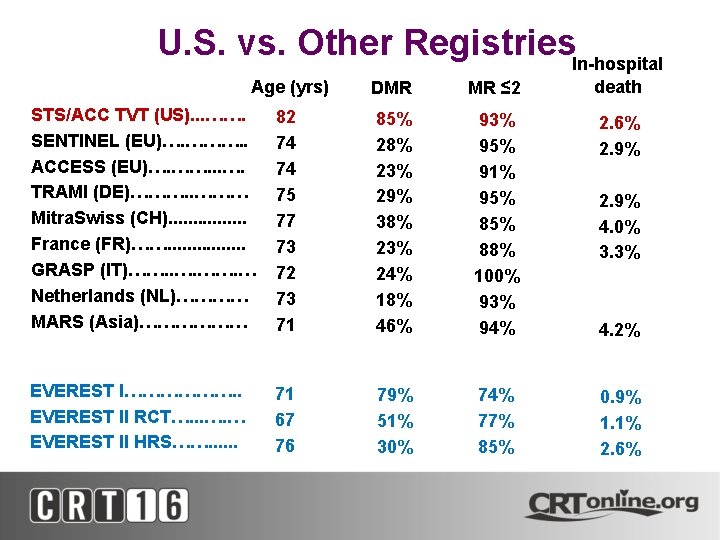
U. S. vs. Other Registries. In-hospital Age (yrs) • • • STS/ACC TVT (US). . . ……. SENTINEL (EU)…. ………. . ACCESS (EU)…. ……. . . …. TRAMI (DE)………. . ……… Mitra. Swiss (CH). . . . France (FR)……. . . . GRASP (IT)……. . …. … Netherlands (NL)………… MARS (Asia)……………… • EVEREST I………………. . • EVEREST II RCT…. . . …. … • EVEREST II HRS……. . . DMR MR ≤ 2 death 82 74 74 75 77 73 72 73 71 85% 28% 23% 29% 38% 23% 24% 18% 46% 93% 95% 91% 95% 88% 100% 93% 94% 2. 6% 2. 9% 71 67 76 79% 51% 30% 74% 77% 85% 0. 9% 1. 1% 2. 6% 2. 9% 4. 0% 3. 3% 4. 2%

OUR EXPERIENCE • SYMPTOMATIC IMPROVEMENT WITH MITRACLIP THERAPY FOR PROHIBITIVELY HIGH-RISK DMR PATIENTS. J Am Coll Cardiol. 2015; 65(10_S). MAINI B ET AL. • IMPROVEMENT IN QUALITY OF LIFE IN PATIENTS WITH DEGENERATIVE MITRAL REGURGITATION ST PROHIBITIVE SURGICAL RISK FOLLOWING TRANSCATHETER MITRAL VALVE REPAIR WITH THE MITRACLIP SYSTEM. J Am Coll Cardiol. 2015; 65(10_S). MAINI B ET AL.

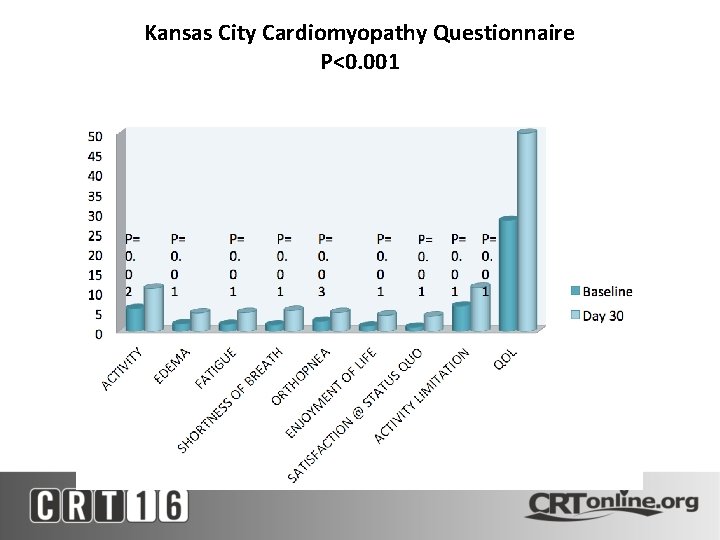
Kansas City Cardiomyopathy Questionnaire P<0. 001

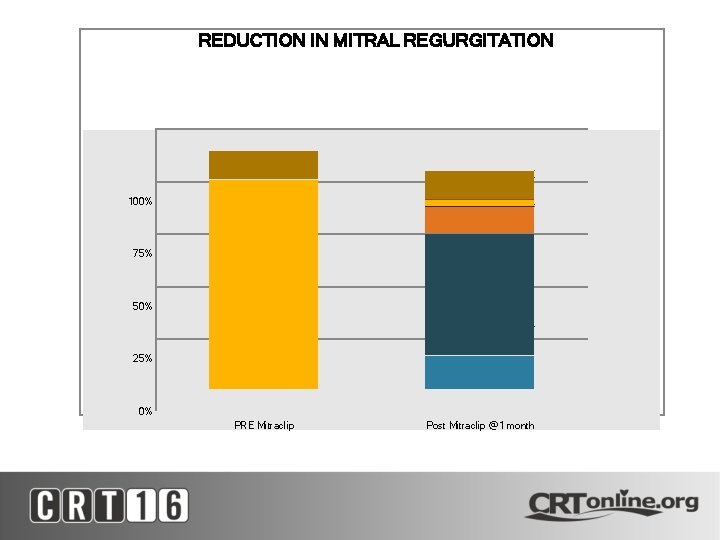
REDUCTION IN MITRAL REGURGITATION 100% 75% 50% 25% 0% PRE Mitraclip Post Mitraclip @1 month

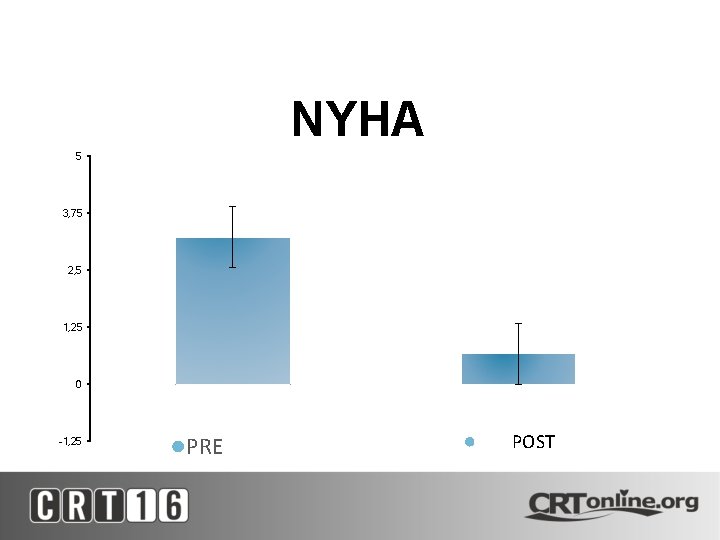
NYHA 5 3, 75 2, 5 1, 25 0 -1, 25 ●PRE ● POST

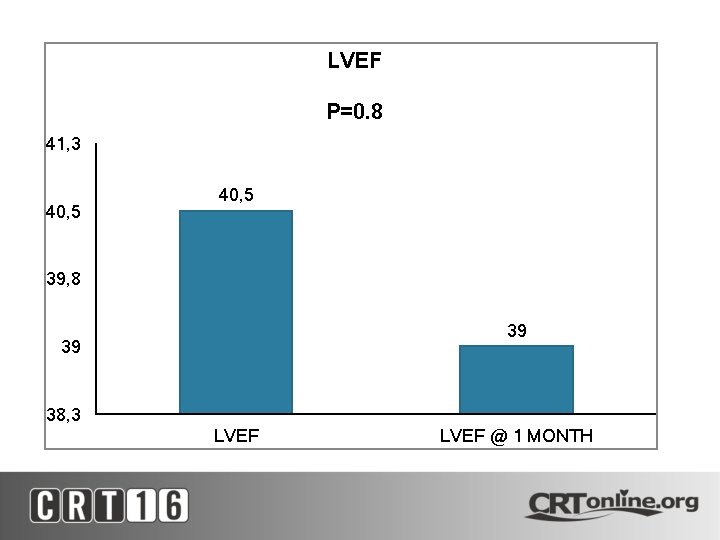
LVEF P=0. 8 41, 3 40, 5 39, 8 39 39 38, 3 LVEF @ 1 MONTH

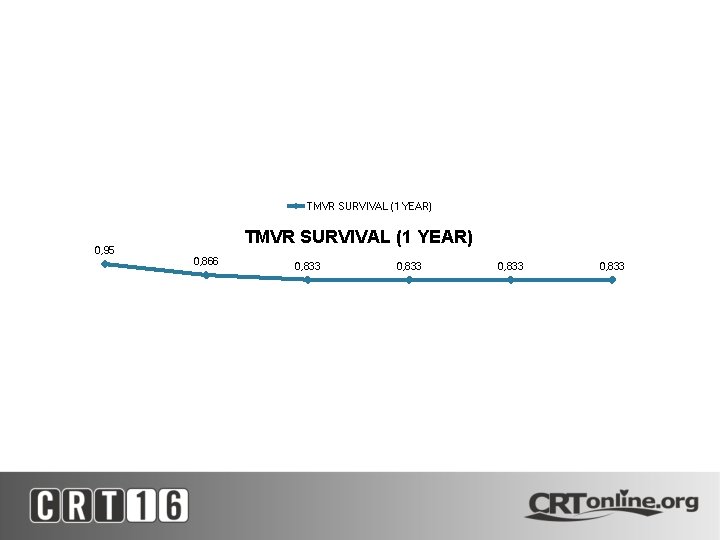
TMVR SURVIVAL (1 YEAR) 0, 95 TMVR SURVIVAL (1 YEAR) 0, 866 0, 833

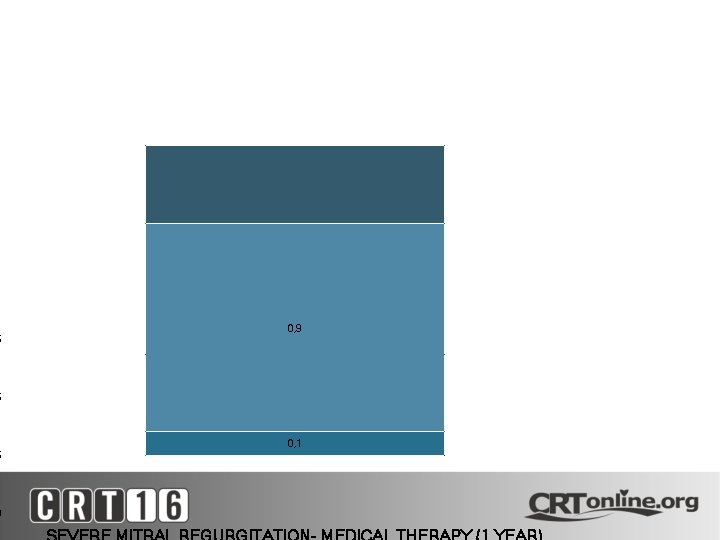
1 5 0, 9 5 5 0 0, 1

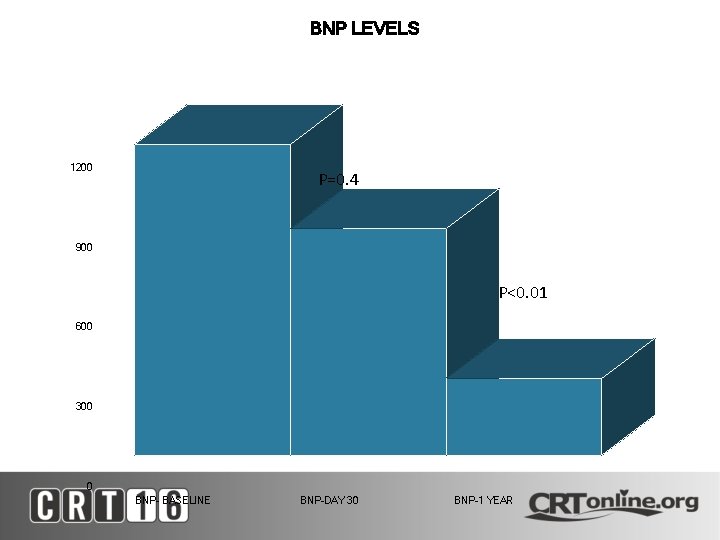
BNP LEVELS 1200 P=0. 4 900 P<0. 01 600 300 0 BNP- BASELINE BNP-DAY 30 BNP-1 YEAR

Conclusions 1. Mitra. Clip is safe and effective for the treatment of prohibitive risk patients with symptomatic MR 2. The commercial outcomes in the U. S. compare favorably to pre-approval studies and other national registries 3. Mitraclip is a therapeutic solution for patients with severe MR– who are not a candidate for surgery