MITOGENACTIVATED PROTEIN KINASE MAPK SIGNAL TRANSDUCTION PATHWAY Ji























- Slides: 31

MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) SIGNAL TRANSDUCTION PATHWAY Jiří Wilhelm

MAP kinases are intermediates in signal transduction pathways that are initiated by many types of surface receptors The targets of MAPK are located within many cellular compartments MAPK provide a physical link in the signal transduction pathway from the cytoplasm to the nucleus

(these are relatively novel and not well desccribed)







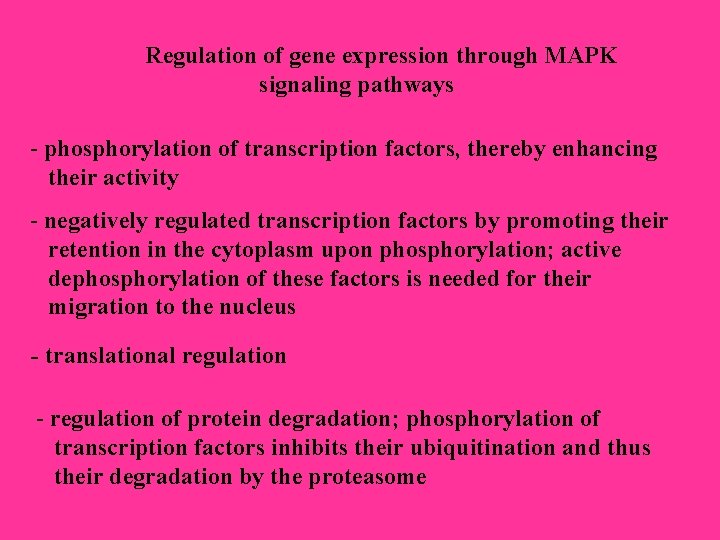
Regulation of gene expression through MAPK signaling pathways - phosphorylation of transcription factors, thereby enhancing their activity - negatively regulated transcription factors by promoting their retention in the cytoplasm upon phosphorylation; active dephosphorylation of these factors is needed for their migration to the nucleus - translational regulation - regulation of protein degradation; phosphorylation of transcription factors inhibits their ubiquitination and thus their degradation by the proteasome

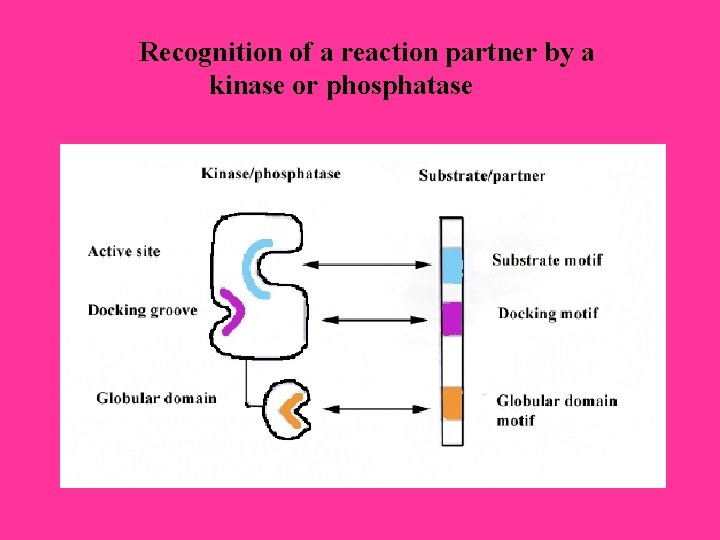
Recognition of a reaction partner by a kinase or phosphatase

NONRECEPTOR TYROSINE KINASES These are cytoplasmic enzymes that play roles in many signal transduction pathways. The term “nonreceptor“ means they lack a transmembrane domain, although they are associated with cell membranes. They are grouped into families: Src Syk Abl Fak Tec/Btk

-Src was the first protein kinase with identified role in malignant transformation. Rous’s Sarcoma Virus contained src gene coding tyrosine kinase. The viral gene is now called v-src, while the cellular proto oncogene is labeled c-src, or just src. Src family kinases function in development and in hematopoietic cells. They are activated by integrin ligands, cytokines, and stimuli that act via immunoreceptors.

N-terminal domain of Src kinases is modified by N-myristoylation, which enables membrane anchoring. Other domains include SH 3 domain, SH 2 domain and kinase domain. There are 2 principal conformational states, indicated as “on” and “off”. In the “off” state SH 2 and SH 3 domains are engaged in intramolecular interaction and the kinase domain is inactive. In the “on” state, the intramolecular interactions are absent, the kinase domain is active, and the SH 2 and SH 3 domains are accessible for binding other proteins. Thus, in this state Src can also act as a scaffold for other proteins.

In the cell, Src kinases are regulated by the balance of kinases and phosphatases acting at both inhibitory and activating phosphorylation sites, and by proteins that bind to their SH 2 and SH 3 domains.

- Syk (spleen tyrosine kinase) family of protein kinases have essential roles in the function and development of T cells, B cells, mast cells, monocytes/ macrophages, and the lymphatic system. - Tec family of PTKs is primarily found in hematopoietic cells. It plays a critical part in T cell or B cell receptor signaling and is also involved in cytokine receptor signaling. These PTKs are functionally interconnected with Src kinases in cell signaling.

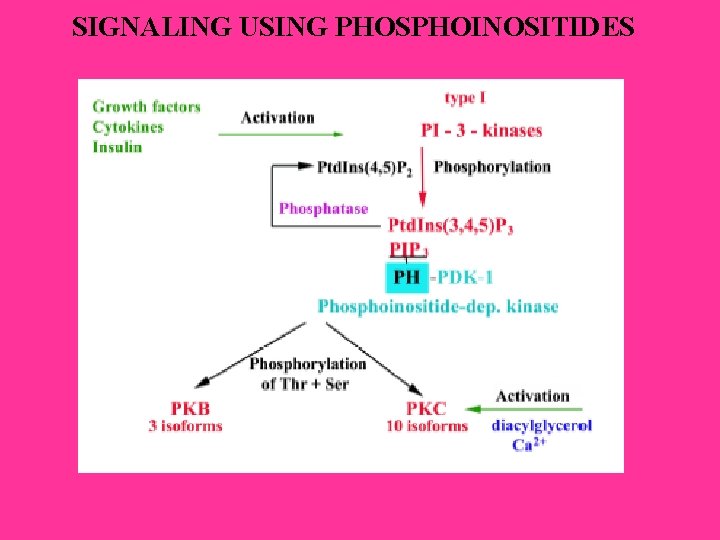
SIGNALING USING PHOSPHOINOSITIDES

Phosphorylated PKC is localized to the cytosol, where it is maintained inactive, because the pseudosubstrate sequence occupies the substrate-binding cavity. The membrane-bound species adopt the active conformation by removal of the pseudo substrate. Correct subcellular location is essential for normal signaling. An abundance of scafold protein that tether PKC near its substrates, activators, and regulatory proteins is needed.

Down-regulation involves dephosphorylation of activated PKC, followed by ubiquitination and proteolysis. The chaperone HSP 70 protects PKC from down-regulation. PKC phosphorylates many substrates, including membrane proteins, cytoskeletal proteins, cytosolic and nuclear proteins. Generally, animals deficient in PKC isozymes are deficient in adaptive responses.


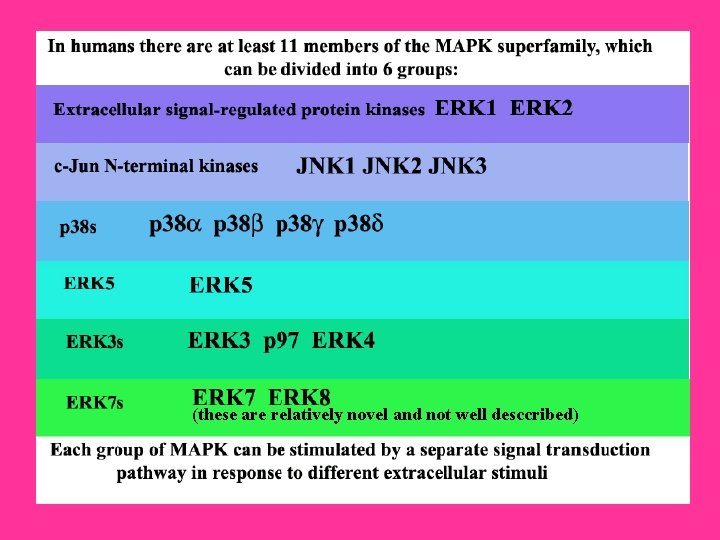
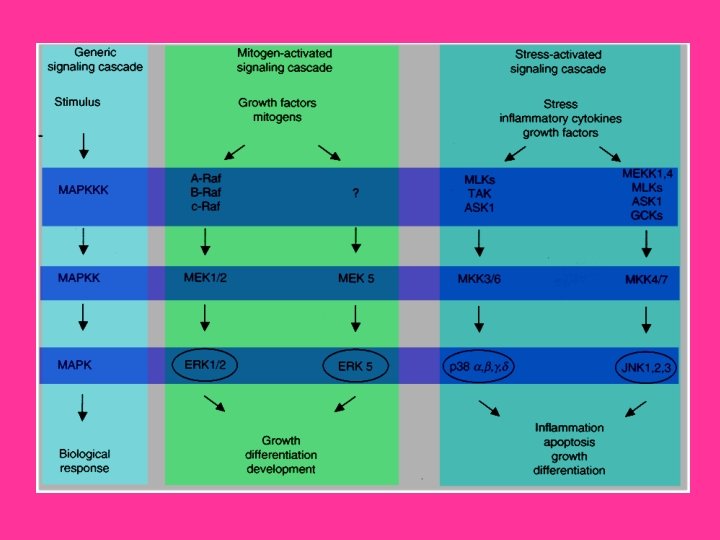
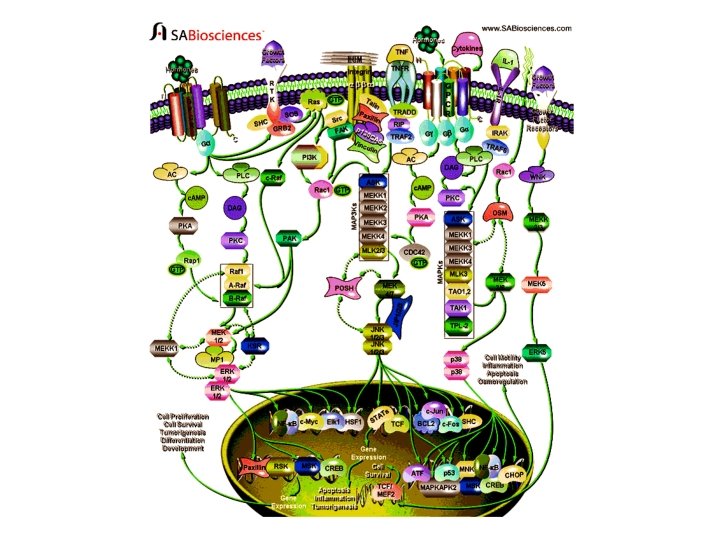
Intracellular signaling cascades are the main routes of communication between the Plasma membrane and regulatory targets in various intracellular compartments. Sequential activation of Kinases is a common mechanism of signal transduction in many cellular processes. During the past decade, several related intracellular signaling cascades have been elucidated, which are collectively known as MAPK (Mitogen. Activated Protein Kinase) signaling cascades. The MAPKs are a group of protein Serine/threonine Kinases that are activated in response to a variety of extracellular stimuli and mediate signal transduction from the cell surface to the nucleus. In combination with several other signaling pathways, they can differentially alter phosphorylation status of numerous proteins, including Transcription Factors, Cytoskeletal proteins, Kinases and other Enzymes, and greatly influence Gene Expression, Metabolism, Cell Division, Cell Morphology and Cell Survival. Furthermore, epigenetic aberrations of these enzymes or of the signaling cascades that regulate them have been implicated in a variety of human diseases including Cancer, Inflammation and Cardiovascular disease. There are four major groups of MAPKs in mammalian cells-the ERKs (Extracellular signal-Regulated Kinases), the p 38 MAPKs, the JNKs (c-Jun NH 2 terminal Kinases) and the ERK 5 (Extracellular signal-Regulated Kinase-5) or BMK cascades. These MAPKs are activated by dual phosphorylation at the tripeptide motif Thr. Xaa-Tyr. The sequence of this tripeptide motif is different in each group of MAPKs: ERK (Thr-Glu-Tyr); p 38 (Thr-Gly-Tyr); and JNK (Thr-Pro-Tyr). Each MAPK pathway contains a three-tiered kinase cascade comprising a MAPKKK/MAP 3 K/MEKK/MKKK (MAP Kinase), a MAPKK/MAP 2 K/MEK/MKK (MAP Kinase) and the MAPK. This three-tier module mediates ultrasensitive switch-like responses to stimuli. Frequently, a MAPKKKK, MAP 4 K or MKKKK (MAPKKK Kinase) activates the MAPKKK. The MAPKKKs then phosphorylates a dual-specificity protein kinase MAPKK, which in turn phosphorylates the MAPK (Ref. 1 & 2).

ERK, the most widely studied MAPK cascade, have been established as a major participant in the regulation of cell growth and differentiation, but when improperly activated contribute to malignant transformation. ERK 1 and ERK 2 form the central component in the ERK cascade. The ERK signaling cascade is activated by a wide variety of receptors involved in growth and differentiation including GPCRs (G-Protein Coupled Receptors), RTKs (Receptor Tyrosine Kinases), Integrins, and Ion Channels. A general activation scheme involves the activation of RTKs by Growth Factors, such as EGF (Epidermal Growth Factor). The subsequent auto-phosphorylation of the cytoplasmic tails of the receptor on tyrosine leads to the tyrosine phosphorylation of the adapter protein SHC can then recruit the GRB 2 (Growth Factor Receptor Bound Protein-2)-SOS (Son of Sevenless protein) complex to the membrane via the SH 2 domain of GRB 2 binding to the phosphotyrosine on SHC. SOS, a GEF for Ras, can then exchange the GDP bound to Ras to GTP. Once Ras binds GTP, it can then recruit the Serine/threonine kinase Raf to the membrane. When Raf translocate to the membrane, it becomes activated and then phosphorylates the dual specificity kinases MKK 1 and MKK 2. The activated MKKs phosphorylate ERK 1/ERK 2 on Threonine 183 and Tyrosine 185 (at the TEY motif) (Ref. 3). GPCR also play an important role in activation of ERKs. When the GPCR becomes activated by Ligands such as Neurotransmitters, Cytokines etc. , it leads to the exchange of GDP for GTP on the GN-Alpha (Guanine Nucleotide -Binding Protein-Alpha) subunit. Upon activation, GN-Alpha. I (Guanine Nucleotide-Binding Protein-Alpha-I) or GN-Alpha. Q (Guanine Nucleotide. Binding Protein-Alpha-Q) subunits are separated from GN-Beta (Guanine Nucleotide-Binding Protein-Beta) and GN-Gamma (Guanine Nucleotide. Binding Protein-Gamma) subunits and are converted to their GTP bound states that exhibit distinctive regulatory features on the nine tm. ACs (Transmembrane Adenylate Cyclases) in order to regulate intracellular c. AMP (Cyclic Adenosine 3', 5'-monophosphate) levels.

c. AMP activate Rap 1 A (Ras-Related Protein-1 A) and Rap 1 B (Ras-Related Protein Rap 1 B) through EPAC (Exchange Protein Activated by c. AMP)dependent pathway. c. AMP activates c. AMP-GEFI (c. AMP-Regulated Guanine Nucleotide Exchange Factor-I)/EPAC 1 and c. AMP-GEFII (c. AMP-Regulated Guanine Nucleotide Exchange Factor-II)/EPAC 2 that in turn activate Rap 1 A and Rap 1 B, respectively. Rap 1 A and Rap 1 B then forms an active complex with BRaf (v-Raf Murine Sarcoma Viral Oncogene Homolog-B 1) for MEK 1/2 activation finally resulting in ERK 1/2 activation. c. AMP may also activate PKA (Protein Kinase-A), which may further activate Rap and thus BRaf. On the other hand, PKA also inactivates C-Raf. GN-Alpha also directly activates PLC (Phospholipase-C) which further activates PKC (Protein Kinase-C) via DAG (Diacylglycerol). PKC further activates Raf and thus ERK. A new mechanism has recently been identified that regulates MEK 1 -ERK interactions and is dependent on Rac and PAK (p 21 -Activated Kinase). Integrins also play an important role in regulating the efficiency of the RTK/Ras/ERK pathway. FAK (Focal Adhesion Kinase) is a major nonreceptor tyrosine kinase activated after Integrin-mediated adhesion to ECM (Extracellular Matrix) proteins such as FN (Fibronectin). Interaction between FAK and the cytoplasmic tail of Beta 1 Integrins results in autophosphorylation of FAK tyrosine 397 (FAK p. Y 397) that can lead to stimulation of a cell-signaling cascade that ultimately activates the Ras/MAPK/ERK pathway. In addition to FAK, members of the Src family of nonreceptor protein-tyrosine kinases also associate with Focal Adhesions and are involved in Integrin signaling.

Interestingly, Src and FAK appear to function in association with each other as a result of the binding of the Src SH 2 domain to an autophosphorylation site of FAK. Src then phosphorylates additional sites on FAK. Tyrosine phosphorylation of FAK creates binding sites for the SH 2 domains of other downstream signaling molecules, including PI 3 K (Phosphatidylinositol 3 Kinase) and Rac. A key target of Rac is the protein-serine/threonine kinase PAK. Rac and CDC 42 (Cell Division Cycle-42) can synergize with Raf to promote activation of the ERKs through mechanisms involving PAK 1 phosphorylation of the MEK 1 proline-rich sequence and PAK 3 phosphorylation of Raf 1. PAK 3 can phosphorylate Raf 1, enhancing Raf 1 activation. Raf 1 finally activates ERK 1/2 via MEK 1/2. ERK once activated translocates to the nucleus to phosphorylate and activate several nuclear targets. The major target of activated ERKs is RSK (90 k. Da Ribosomal protein S 6 Kinase). Active RSKs appear to play a major role in transcriptional regulation, translocating to the nucleus and phosphorylating such factors as the product of proto-oncogene c-Fos at Ser 362, SRF (Serum Response Factor) at Ser 103, and CREB (Cyclic AMP Response Element. Binding protein) at Ser 133. ERK also translocates to the nucleus to phosphorylate transcription factor Elk 1 (on Serine 383 and Serine 389). Another important target of ERK is NF-Kappa. B (Nuclear Factor-Kappa. B), which binds to its consensus sequence (5'-GGGACTTTC-3') and positively regulates the transcription of genes involved in immune and inflammatory responses, cell growth control, and apoptosis. Other nuclear targets of ERK include the MSKs (Mitogen- and Stress-activated protein Kinases), CREB, c. Myc, HSF 1 (Heat-Shock Factor-1), Paxillin and many more transcription factors (Ref. 4, 5 & 6).

Recently, another related kinase, ERK 3, a nuclear protein kinase, has been cloned and is reported to exhibit about 50% homology to ERK 1/ERK 2 within its catalytic domain. However, it does not phosphorylate any typical ERK substrates. The phosphorylation site motif in the activation loop of ERK 3 has a single phosphorylation site located at Serine 189. Another member of ERK family is the ERK 5 that contains at least ten consensus sites for MAPK phosphorylation and may be associated with keeping ERK 5 in high active state. ERK 5 can be activated by proliferative stimuli such as EGF, Serum, Lysophosphatidic acid, Neurotrophins and Phorbol ester, as well as by stress stimuli such as Sorbitol, H 2 O 2, and UV irradiation. WNK 1 (WNK Lysine deficient protein Kinase-1) is required for activation of ERK 5 by EGF. MEK 5 (MAPK/ERK Kinase-5) and MEKK 2/3 (MAP/ERK Kinase-2/3) acts as upstream regulators of ERK 5. The known ERK 5 substrates include the MEF 2 (Myocyte Enhance Factor-2) family members, MEF 2 A, C and D, and the ETS-like transcription factor SAP 1 A (Signaling lymphocytic Activation molecule associated Protein-1 A) (Ref. 7 & 8). The second most widely studied MAPK cascade is the JNK/SAPK (Stress Activated Protein Kinase). The JNKs/ SAPKs are encoded by at least three genes: SAPKAlpha/JNK 2, SAPK-Beta/JNK 3, and SAPK-Gamma/JNK 1. This cascade is activated following exposure to UV radiation, Heat shock, or Inflammatory Cytokines. Directly upstream of JNK, at the MAPKK level, there are two dual specificity kinases that phosphorylate and activate JNK at Serine and threonine residues. These kinases are MKK 4 (MAPK Kinase-4), and MKK 7 (MAPK Kinase-4). These proteins are activated, in turn, by the upstream MAP 3 K: MEKKs (MAPK/ERK Kinases), MLK 2/3 (Mixed Lineage Kinase-2/3), TAK 1 (TGF-Beta-Activated Kinase-1), TPL 2 (Tumor Progression Locus-2), ZPK (Zipper Protein Kinase), and ASK 1 (Apoptosis Signal-regulating Kinase-1). Some other MAP 3 Ks have also been identified, whose functions are not known. These included MAP 3 K 6, MLK 1 (Mixed Lineage Kinase-1) and LZK (Leucine Zipper-bearing Kinase).

The Rho family GTPases, CDC 42 and Rac initiate a cascade leading to JNK/SAPK, presumably by binding and activating the protein kinase PAK, a kinase that phosphorylates and promotes activation of MEKK 1. CDC 42 can also be activated by GPCR. Stimulation of GPCRs coupled to the GN-Alpha. S subunit of trimeric G-proteins, induces production of c. AMP and activation of PKA. Activation of PKA enhances the activity of CDC 42 and thus plays an important role in activation of JNKs. The activation of JNK by Cytokine receptors appears to be mediated by the TRAF (TNF Receptor-Associated Factor) group of Adaptor proteins. Activation of the TNFR (Tumor necrosis Factor Receptor) leads to recruitment of TRAF 2 (TNF Receptor-Associated Factor-2), which is required for JNK activation. This Adaptor protein (TRADD (Tumor Necrosis Factor Receptor-1 -Associated Death Domain Protein), RIP (Receptor-Interacting Protein), Daxx) has been reported to bind MEKK 1 and ASK 1. The activated JNK/SAPKs translocate to the nucleus where they phosphorylate transcription factors such as c-Jun, c-Fos, DPC 4 (Deleted in Pancreatic Carcinoma 4), p 53, ATF 2 (Activating Transcription Factor-2), NFAT 4 (Nuclear Factor of Activated T-Cell-4), NFAT 1 (Nuclear Factor of Activated T-Cell-1), STAT 1 (Signal Transducers and Activators of Transcription-1), HSF 1, SHC and Bcl 2 (B-Cell CLL/Lymphoma-2). JNKregulated transcription factors help to regulate gene expression in response to a variety of cellular stimuli, including stress events, Growth Factors and Cytokines. Activation of the JNK signaling cascade generally results in Apoptosis, although it has also been shown to promote cell survival under certain conditions and has important roles in determining cell fate during metazoan development as well as involvement in tumorigenesis and inflammation (Ref. 9, 10 & 11).

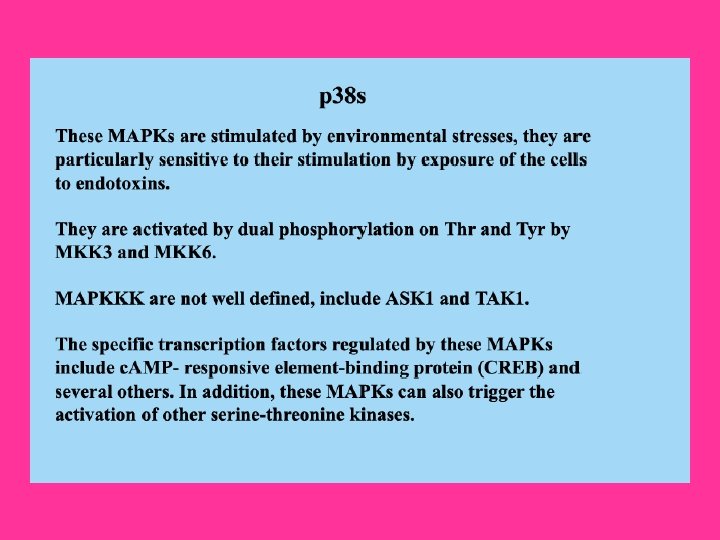
The p 38 kinase is most well-characterized member of the MAP kinase family. It shares about 50% homology with the ERKs. Four p 38 MAPKs have been cloned so far in higher eukaryotes: p 38 -Alpha/XMpk 2/CSBP, p 38 -Beta/p 38 -Beta 22, p 38 -Gamma/SAPK 3/ERK 6, and p 38 -Delta/SAPK 4. The mammalian p 38 MAPK families are activated by cellular stress including UV irradiation, Heat shock, High osmotic stress, Lipopolysaccharide, Protein synthesis inhibitors, Proinflammatory Cytokines (such as IL-1 (Interleukin-1) and TNF-Alpha (Tumor Necrosis Factor-Alpha)) and certain Mitogens. The upstream MAPK cascade in p 38 activation includes MAPKKKs such as ASK 1, MEKK 4, MLK 2 and 3, DLK (Dual Leucine Zipper Kinase), TPL 2 (Tumor Progression Locus-2), TAK 1 and TAO 1/TAO 2, which phosphorylate and activate MKK 3 and MKK 6, which in turn phosphorylate and activate p 38. Proinflammatory cytokines such as IL and TNF are the main stimulator of p 38. IL-1 signaling is known to involve PI 3 K, p 38 MAPK and ERK. After IL-1 is bound to its receptor IL-1 R (IL-1 Receptor), a complex is formed between the Type-1 Receptor and the receptor accessory protein. The cytosolic proteins My. D 88 (Myeloid Differentiation primary response gene-88) and Toll. IP (Toll-Interacting Protein) are recruited to this complex, where they function as adaptors, recruiting IRAK 1 (IL-1 Receptor-Associated Kinase-1) in turn. IRAK 1, a serine-threonine kinase, activates and recruits TRAF 6 (TNF Receptor-Associated Factors-6) to the IL-1 receptor complex. Eventually, phosphorylated IRAK is ubiquitinated and degraded. TRAF 6 signals through the TAB 1 (TAK 1 Binding Protein-1)/TAK 1 (TGF-Beta-Activating Kinase-1) kinases to activate MKKs, which further activates p 38 MAPK (Ref. 12).

TNF also stimulate p 38 signaling. Binding of TNFR 1 to TNF-Alpha results in conformational changes in the receptor's intracellular domain, resulting in rapid recruitment of several cytoplasmic death domain-containing adapter proteins via homophilic interaction with the death domain of the receptor. The first adaptor recruited to the clustered receptor is the TNFR-associated protein with death domain, which functions as a docking protein for several signaling molecules, such as FADD (Fas. Associated protein with Death Domain), TRADD, Daxx, TRAF 2 and RIP associates with TRAF 2 to generate MEKK 4 and ASK 1. Both MEKK 4 and ASK 1 activates p 38 MAPKs by activating MKK 3 and MKK 6. Besides, p 38 can also be activated by GPCRs and numerous physical and chemical stresses, including hormones, UV irradiation, ischemia, osmotic shock and heat shock. G-proteins activate p 38 via PKA or PKC, whereas stress activates p 38 via Rac and CDC 42. Following its activation, p 38 translocates to the nucleus and phosphoryates ATF 2. Another known target of p 38 is MAPKAPK 2 (MAPK-Activated Protein Kinase-2) that is involved in the phosphorylation and activation of heat-shock proteins. Other transcription factors affected by the p 38 family include STAT 1 (Signal Transducers and Activators of Transcription-1), Max/Myc complexes, Elk 1 and CREB through the activation of MSK 1 (Mitogen- and Stress-Activated Kinase-1). The p 38 subfamily is also involved in affecting Cell Motility, Transcription and Chromatin Remodeling. Other substrates of the p 38 signaling pathway include CHOP (C/EBPHomologous Protein) for regulation of gene expression, as well as MNK 1 (MAPKInteracting Kinase-1). p 38 MAPK is a crucial mediator in the NF-kappa. B-dependent gene activation induced by TNF (Ref. 13, 14 & 15).

The mammalian MAPK signaling system employ scaffold proteins, in part, to organize the MAPK signaling components into functional MAPK modules, thereby enabling the efficient activation of specific MAPK pathways. The ERK scaffold protein KSR (Kinase Suppressor of Ras) binds ERK, its direct activator MEK and Raf. A second targeting protein, p 14, targets ERK 2 to an endosomal location through its interaction with MP 1 (MAPKK 1 Interacting Protein-1), an adaptor protein that binds MEK and ERK. In addition, MEKK 1 (MAP/ERK Kinase-1) can serve both as a scaffold and as MAPKKK, interacting specifically with MAPKK and MAPK. Multidomain protein Posh (Plenty of SH 3 s) acts as a scaffold for the JNK pathway. Posh binds MLKs both in vivo and in vitro, and complexes with MKKs 4 and 7 and with JNKs. The JNK MAPK modules are also regulated by a JIP 1 (JNK Interacting Protein-1), JIP 2 (JNK Interacting Protein-2), JIP 3 (JNK Interacting Protein-3), JIP 4 (JNK Interacting Protein-4), Beta-Arrestin-2, Filamin and Crk. II. There is increasing evidence that the three well-characterized members of the MAPK family, ERK 1/2, JNK/SAPK and p 38 play an important role in regulation of proliferation in mammalian cells by sharing substrate and cross-cascade interaction. MAPK pathways are involved in many pathological conditions, including cancer and other diseases. Therefore, a better understanding of the relationship between MAP kinase signal transduction system and the regulation of cell proliferation is essential for the rational design of novel pharmacotherapeutic approaches (Ref. 16 & 17).

1. Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004 Apr 12; 23(16): 2838 -49. 2. Panka DJ, Atkins MB, Mier JW. Targeting the mitogen-activated protein kinase pathway in the treatment of malignant melanoma. Clin Cancer Res. 2006 Apr 1; 12(7 Pt 2): 2371 s-2375 s. 3. Subramaniam S, Unsicker K. Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience. 2006; 138(4): 1055 -65. Epub 2006 Jan 25. 4. Osmond RI, Sheehan A, Borowicz R, Barnett E, Harvey G, Turner C, Brown A, Crouch MF, Dyer AR. GPCR screening via ERK 1/2: a novel platform for screening G protein-coupled receptors. J Biomol Screen. 2005 Oct; 10(7): 730 -7. Epub 2005 Aug 29. 5. Ginnan R, Guikema BJ, Singer HA, Jourd'heuil D. PKC{delta} mediates activation of ERK 1/2 and induction of i. NOS by Interleukin-1{beta} in Vascular Smooth Muscle Cells. Am J Physiol Cell Physiol. 2006 Jan 25; 6. Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol Biotechnol. 2005 Jan; 29(1): 57 -74. 7. Wang X, Tournier C. Regulation of cellular functions by the ERK 5 signalling pathway. Cell Signal. 2006 Jun; 18(6): 753 -60. Epub 2006 Jan 6. 8. Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The MAP kinase ERK 5 binds to and phosphorylates p 90 RSK. Arch Biochem Biophys. 2006 May 15; 449(1 -2): 8 -16. Epub 2006 Mar 13. 9. Yang Q, Kim YS, Lin Y, Lewis J, Neckers L, Liu ZG. Tumour necrosis factor receptor 1 mediates endoplasmic reticulum stress-induced activation of the MAP kinase JNK. EMBO Rep. 2006 May 5; 10. Yamauchi J, Miyamoto Y, Kokubu H, Nishii H, Okamoto M, Sugawara Y, Hirasawa A, Tsujimoto G, Itoh H. Endothelin suppresses cell migration via the JNK signaling pathway in a manner dependent upon Src kinase, Rac 1, and Cdc 42. FEBS Lett. 2002 Sep 11; 527(1 -3): 284 -8.

Heasley LE, Han SY. JNK Regulation of Oncogenesis. Mol Cells. 2006 Apr 30; 21(2): 167 -73. Mittelstadt PR, Salvador JM, Fornace AJ Jr, Ashwell JD. Activating p 38 MAPK: new tricks for an old kinase. Cell Cycle. 2005 Sep; 4(9): 1189 -92. Epub 2005 Sep 20. Vergarajauregui S, San Miguel A, Puertollano R. Activation of p 38 Mitogen-Activated Protein Kinase Promotes Epidermal Growth Factor Receptor Internalization. Traffic. 2006 Jun; 7(6): 686 -98. Kaur R, Liu X, Gjoerup O, Zhang A, Yuan X, Balk SP, Schneider MC, Lu ML. Activation of p 21 -activated kinase 6 by MAP kinase 6 and p 38 MAP kinase. J Biol Chem. 2005 Feb 4; 280(5): 3323 -30. Epub 2004 Nov 18. Lokuta MA, Huttenlocher A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p 38 MAPK pathway. J Leukoc Biol. 2005 Jul; 78(1): 210 -9. Epub 2005 Apr 21. Kortum RL, Costanzo DL, Haferbier J, Schreiner SJ, Razidlo GL, Wu MH, Volle DJ, Mori T, Sakaue H, Chaika NV, Chaika OV, Lewis RE. The molecular scaffold kinase suppressor of Ras 1 (KSR 1) regulates adipogenesis. Mol Cell Biol. 2005 Sep; 25(17): 7592 -604. Moulin N, Widmann C. Islet-brain (IB)/JNK-interacting proteins (JIPs): future targets for the treatment of neurodegenerative diseases? Curr Neurovasc Res. 2004 Apr; 1(2): 111 -27. Copyright © 1998 - 2009 SABiosciences Corporation