MiniUnit 12 Reaction Rates and Equilibrium and Le

Mini-Unit 12: Reaction Rates and Equilibrium and Le. Châtelier’s Principle

Equilibrium (Eq) Equilibrium occurs whenever two opposite reactions occur at the same rate. Example: 2 SO 2 + O 2 ⇌ 2 SO 3
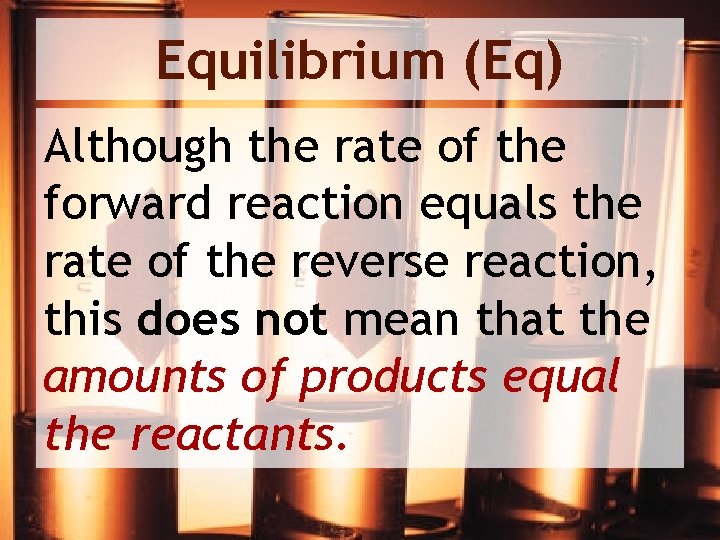
Equilibrium (Eq) Although the rate of the forward reaction equals the rate of the reverse reaction, this does not mean that the amounts of products equal the reactants.

Le Châtelier’s Principle: If a stress is applied to a system in equilibrium, the reaction will shift in the direction to relieve that stress.

(1) Change in Concentration An increase in concentration causes the system to shift in the direction that will use that substance up. A decrease in concentration causes the system to shift in the direction that will make more of that substance.

Example: 2 SO 2(g) + O 2(g) ⇌ 2 SO 3(g) shift right [SO 2], _____ shift left [SO 3], _____ [O 2], _____ shift left Note: Changing the concentration of a pure solid or pure liquid has no effect on the equilibrium.
![Example: 2 Na(s) + Cl 2(g) ⇌ 2 Na. Cl(s) [Na. Cl], _____ no Example: 2 Na(s) + Cl 2(g) ⇌ 2 Na. Cl(s) [Na. Cl], _____ no](http://slidetodoc.com/presentation_image/af407977a723caf63b582740dafad807/image-7.jpg)
Example: 2 Na(s) + Cl 2(g) ⇌ 2 Na. Cl(s) [Na. Cl], _____ no change [Na], _____
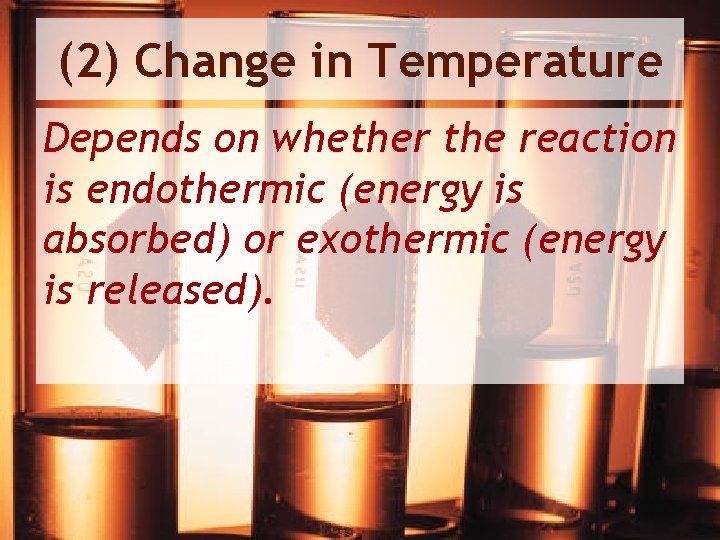
(2) Change in Temperature Depends on whether the reaction is endothermic (energy is absorbed) or exothermic (energy is released).
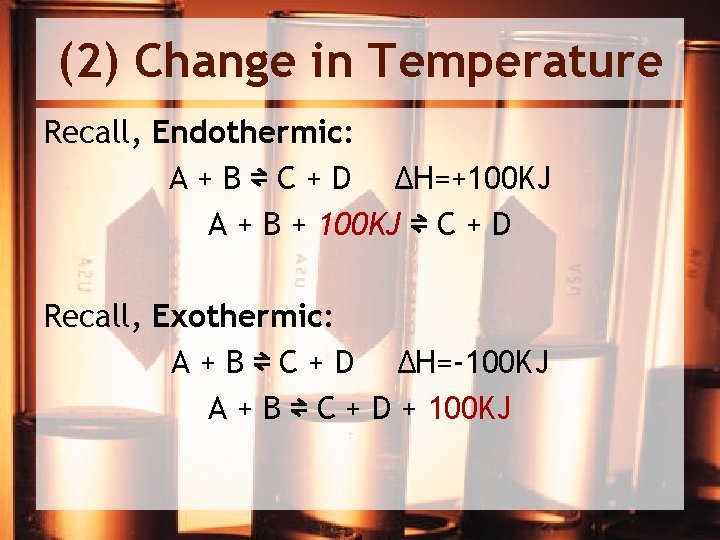
(2) Change in Temperature Recall, Endothermic: A + B ⇌ C + D ΔH=+100 KJ A + B + 100 KJ ⇌ C + D Recall, Exothermic: A + B ⇌ C + D ΔH=-100 KJ A + B ⇌ C + D + 100 KJ

Example: 2 SO 2(g) + O 2(g) ⇌ 2 SO 3(g) + 135 k. J Add. Remove heat at e h eat uct! r T nt: r prod i h ful ant o p l He react as a temp, shift left _______ temp, shift right _______
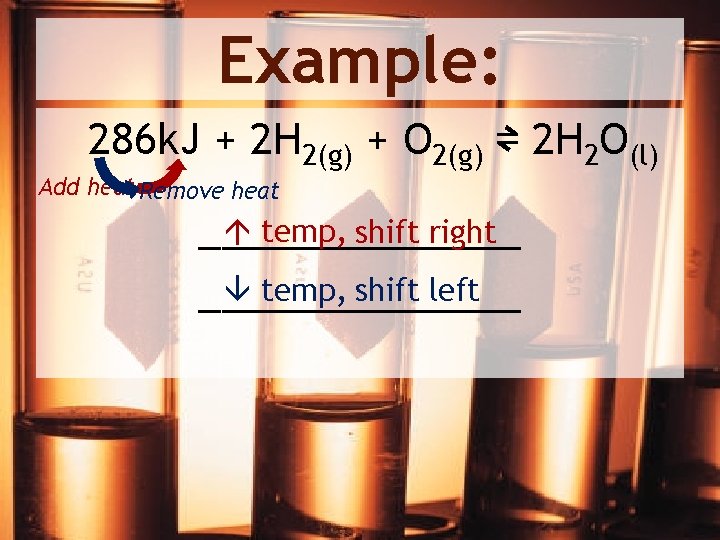
Example: 286 k. J + 2 H 2(g) + O 2(g) ⇌ 2 H 2 O(l) Add heat Remove heat temp, shift right _______ temp, shift left _______

(3) Change in Pressure Affects gases only. Increase pressure: Will shift toward the side with less moles of gas.
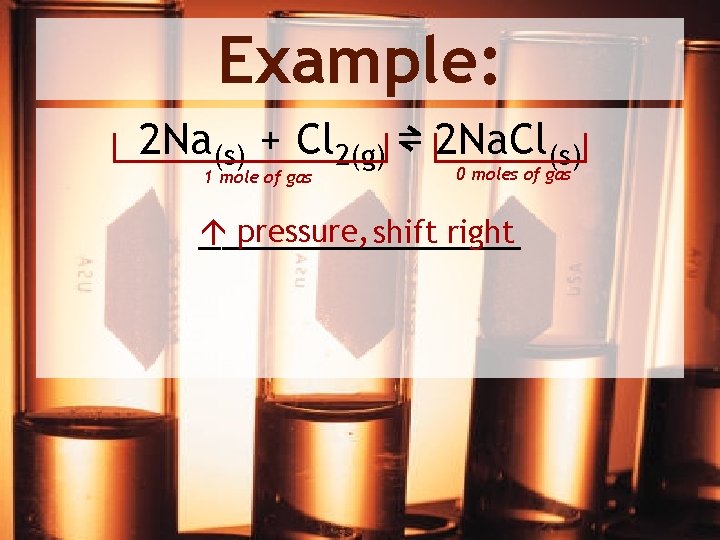
Example: 2 Na(s) + Cl 2(g) ⇌ 2 Na. Cl(s) 1 mole of gas 0 moles of gas pressure, shift right _______

(3) Change in Pressure Decrease pressure: Will shift toward the side with more moles of gas.
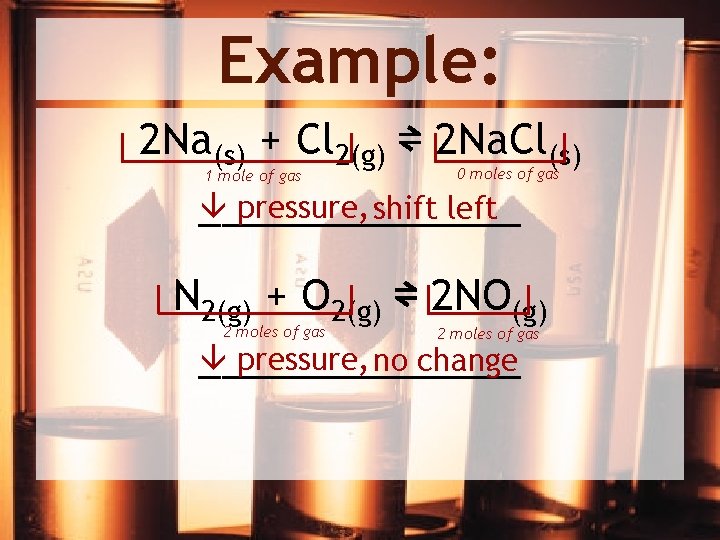
Example: 2 Na(s) + Cl 2(g) ⇌ 2 Na. Cl(s) 1 mole of gas 0 moles of gas pressure, shift left _______ N 2(g) + O 2(g) ⇌ 2 NO(g) pressure, no change _______ 2 moles of gas
(4) Addition of a Catalyst Because a catalyst increases the rate of a reaction, it will have no change on the equilibrium.
- Slides: 16