Mining And its environmental impact Outline n 1












































- Slides: 57

Mining And its environmental impact

Outline n 1. Types of Mining (and why we use them) n 2. Beneficiation n 3. Smelting n 4. Environmental Concerns of 1 through 3

What determines the type of mining? n Underground Solution v. s. Surface Mining v. s. – Depth of below surface – Size of the ore body – Shape of the ore body – Grade – Type of Ore

Depth and Size

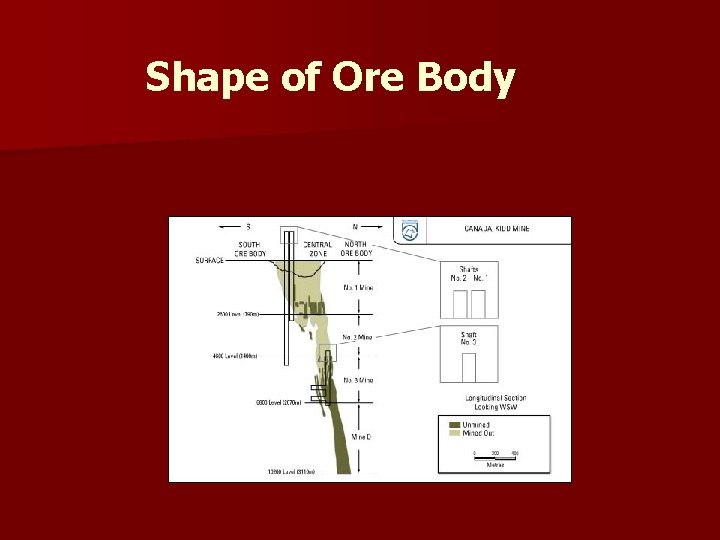
Shape of Ore Body

versus

Type of Ore n Is the ore mineral soluble in water? n Can the ore be melted?

What are the types of mining? n Surface – Strip – Open Pit – Placers--Dredging n Underground n Solution

When do you use Surface Mining? n Large tonnage n High rates of production n Overburden (including rock) is thin

Strip Mining of Coal Kansas Geological Survey

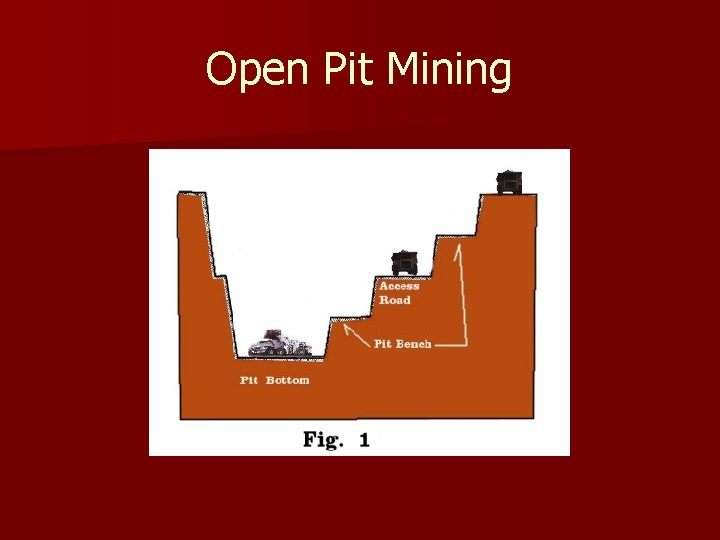
Open Pit Mining



Some photos and machinery used in open-pit mining


? Dinky Toy?

Drilling in pit

Crushing in pit

Loading ore in pit

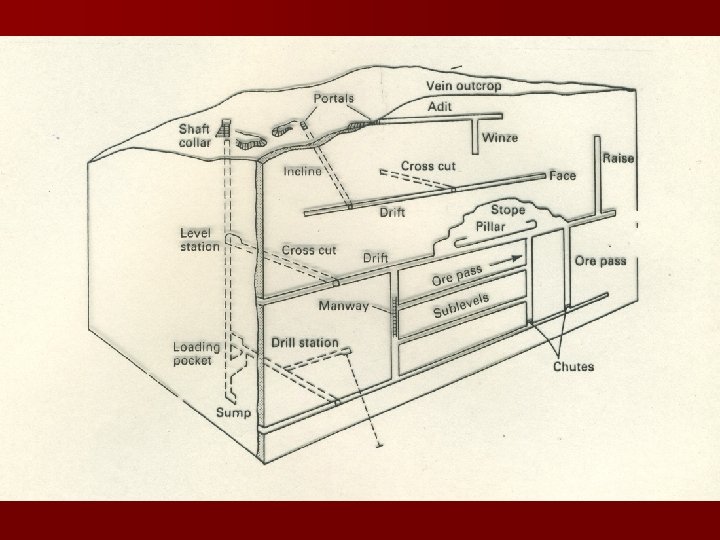
Underground Mining

When do we mine underground? n The ore deposit is deep n Ore body is steep n Grade is high enough to cover costs


Some types of underground mining n Room and Pillar n Cut and Fill n Long wall (coal) n Shrinkage Stoping n Block Caving

Room and Pillar

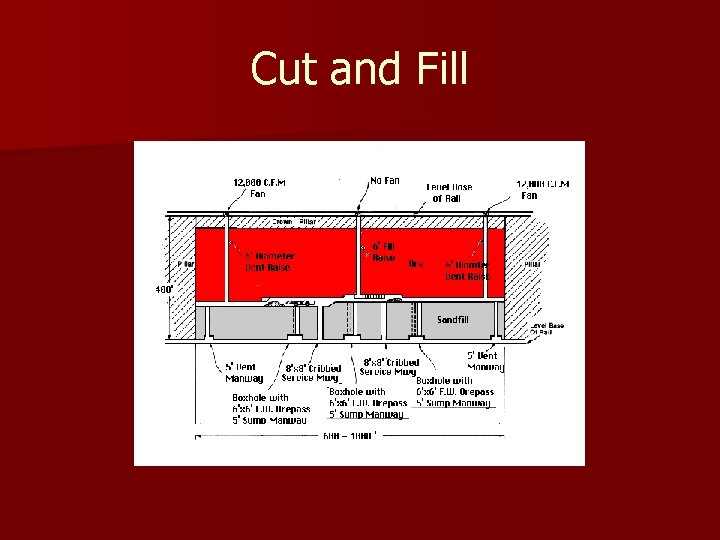
Cut and Fill

Long Wall

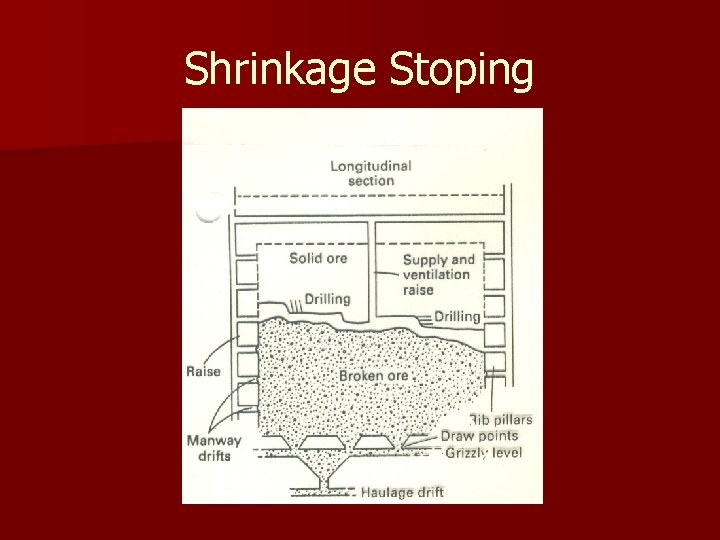
Shrinkage Stoping

Block Caving www. ivanhoe-mines. com/s/Mongolia_Image. Gallery

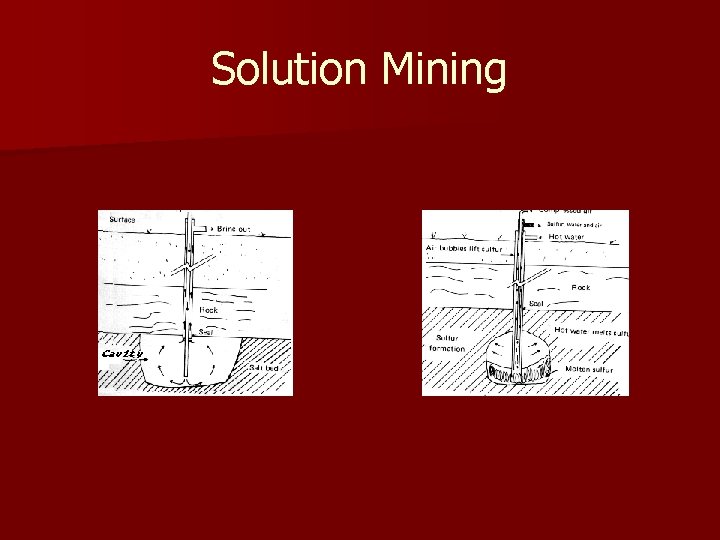
Solution Mining

Beneficiation Means of separation of ore mineral from waste material (or gangue minerals) Also known as Liberation

What does it entail? n Crushing and Grinding – Ball mill or rod mill n Separation – Density (e. g. diamonds with a jig) – Magnetic properties – Electric properties – Surface properties





Refining the Ore Smelting removes the metal from the ore mineral by a variety of ways Heap Leaching removes metal from the ore by solution

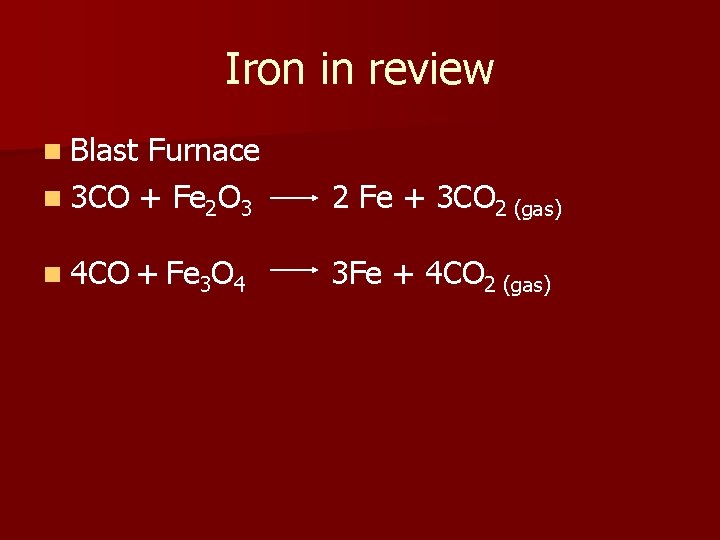
Iron in review n Blast Furnace n 3 CO + Fe 2 O 3 2 Fe + 3 CO 2 (gas) n 4 CO + Fe 3 O 4 3 Fe + 4 CO 2 (gas)

Sulphide Minerals n Are sometimes roasted – Heated in air without melting to transform sulphides to oxides – Gives off H 2 S and SO 2 – Then oxides processed like Fe

Sulphides cont’d n Process of roasting and smelting together creates a matte – Sulfides are melted into a matte and air is blown through. S is converted to sulfur dioxide and Fe to iron oxide, and Cu and Ni stay in melt

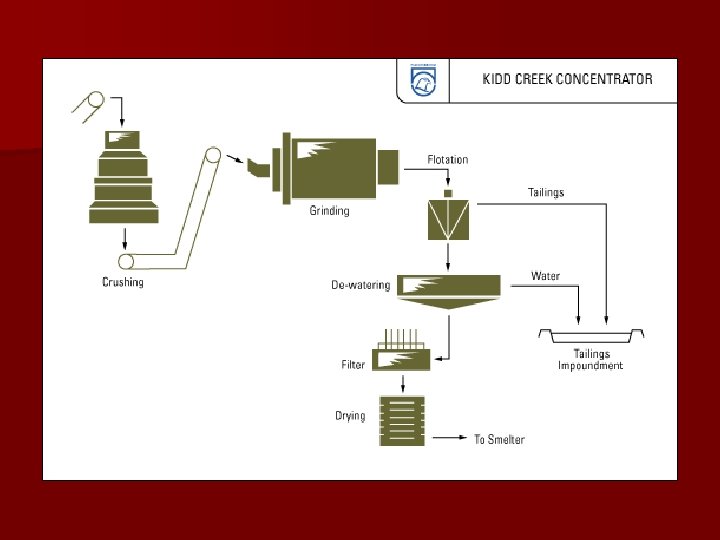
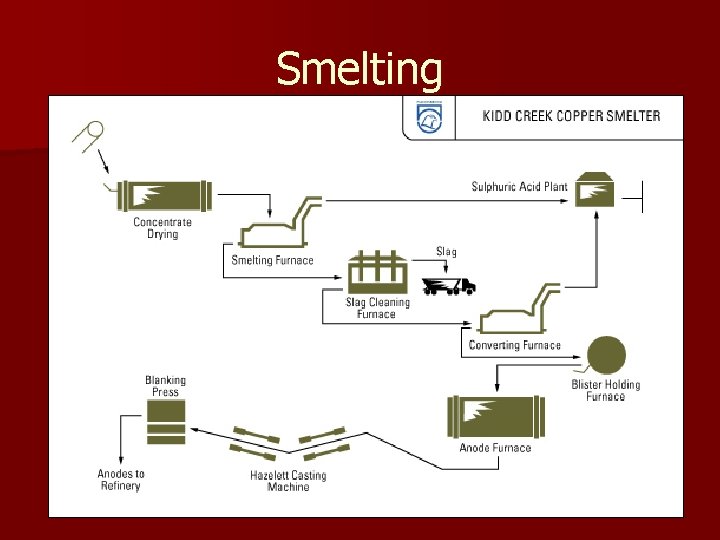
Smelting

Result at Kidd Creek

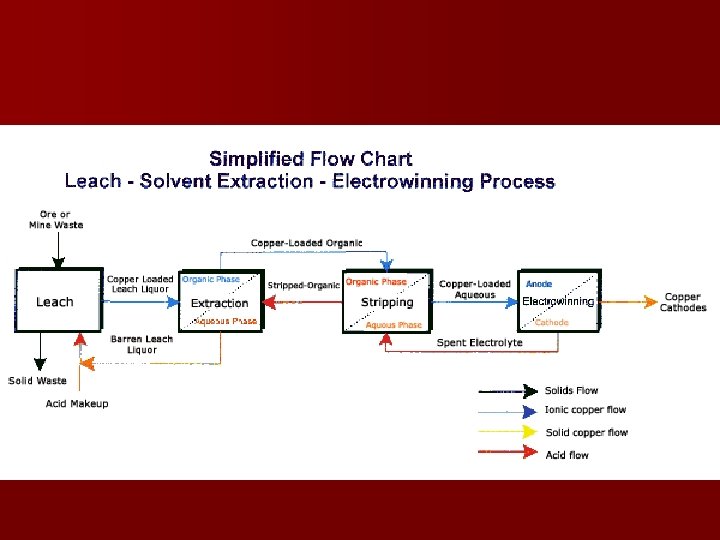
Sulphides cont’d n Solvent extraction/electroplating – Used where rock contains Cu but in too little amounts to be recovered by classical methods


Heap Leaching n In this process, typically done for Au, the ore is not ground, but rather, crushed and piled on the surface. n Weak solutions of Na. CN (0. 05%) percolate through the material leaching out the desired metals. n The solutions are collected and the metals are precipitated

Potential Environmental Problems n A. Mining operation itself – Disposal of a large amount of rock and waste – Noise – Dust n Beneficiation n Smelting and refining

From Underground Mining n Subsidence – Block/caving – Room and pillar – Salt mining (Droitwich)

Subsidence in rancher’s field

Subsidence from Pb-Zn mining

From Underground n Acid Mine Drainage – Fe. S minerals in coal – Sulphide deposits – Acidic streams can pick up heavy elements and transport them

Rock that has acid forming material

Drainage

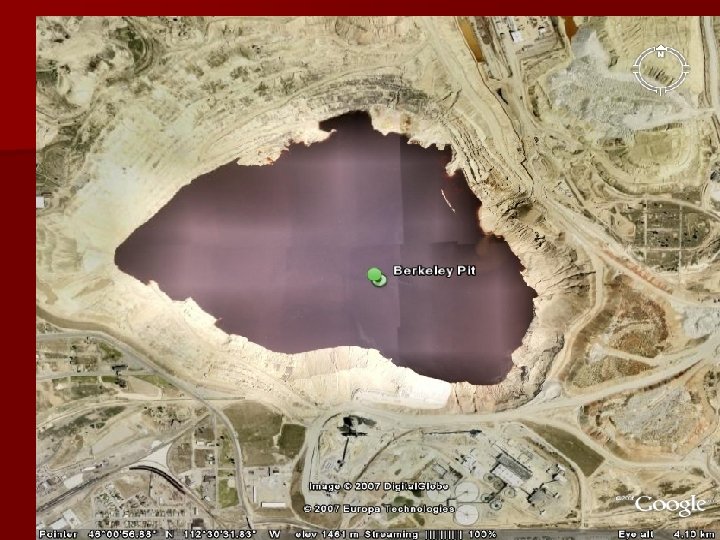
Acid and open pits Berkley Pit


Other problems with open pits n Very large holes n Pit slopes steep and not stable. Cannot be maintained n May fill with water n Strip coal mines –loss of top soil in past – Now smoothed out and top soil added

Disposal of Waste Rock n More problematic for open pit than underground n Waste rock piles have steep angle of repose and thus may not be stable n Bingham in its hay day produced 400, 000 tons of waste rock per DAY!

Tailings ponds n From concentrating usually have high p. H – At Bingham acid waters mixed with tailings water to neutralize n Different metals have different problems

Problems with Smelting/Roasting n Air: SO 2 and CO 2 and particulate matter n Noranda Quebec used to have the highest single point source of SO 2 in the world. It may have been surpassed. n CN (Au); Na. OH and F (Al); solvents (electrotwinning); heavy metals; oil and grease