Mini Quiz 8 Problem Complete this reaction equation
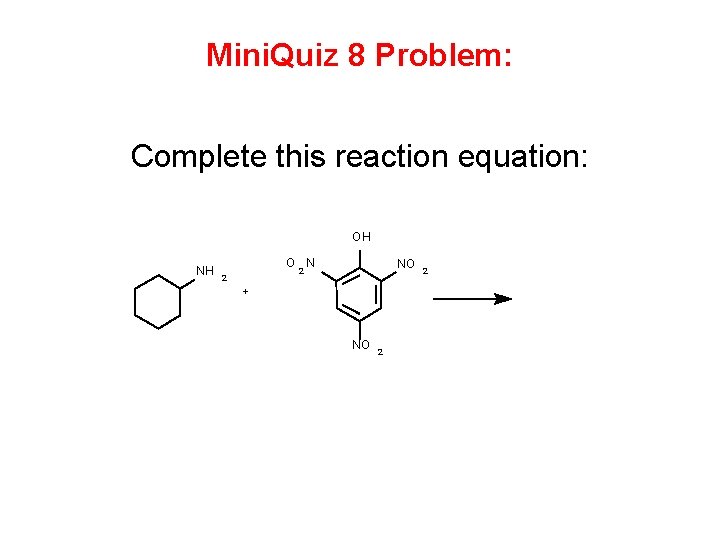
Mini. Quiz 8 Problem: Complete this reaction equation: OH NH O N NO 2 2 + NO 2 2
Today: Mass Spectrometry Next time: H-NMR, CMR Following Tuesday (May 12): Your questions Final Exam in Lab
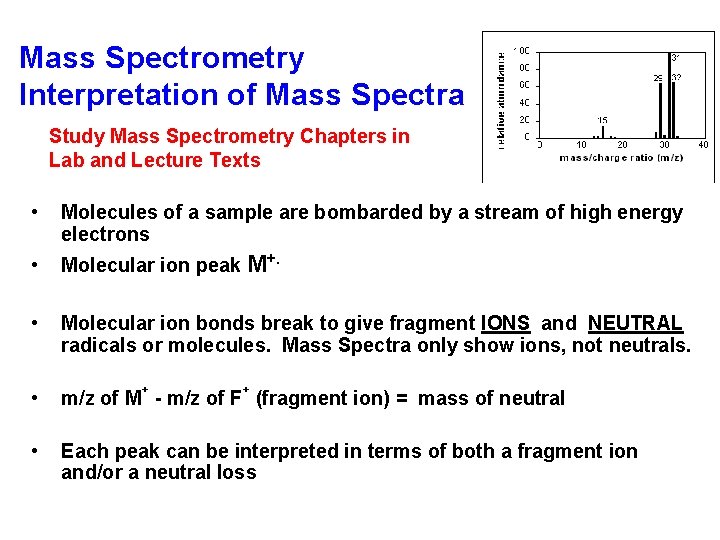
Mass Spectrometry Interpretation of Mass Spectra Study Mass Spectrometry Chapters in Lab and Lecture Texts • Molecules of a sample are bombarded by a stream of high energy electrons • Molecular ion peak M+. • Molecular ion bonds break to give fragment IONS and NEUTRAL radicals or molecules. Mass Spectra only show ions, not neutrals. • m/z of M - m/z of F (fragment ion) = mass of neutral • Each peak can be interpreted in terms of both a fragment ion and/or a neutral loss + +
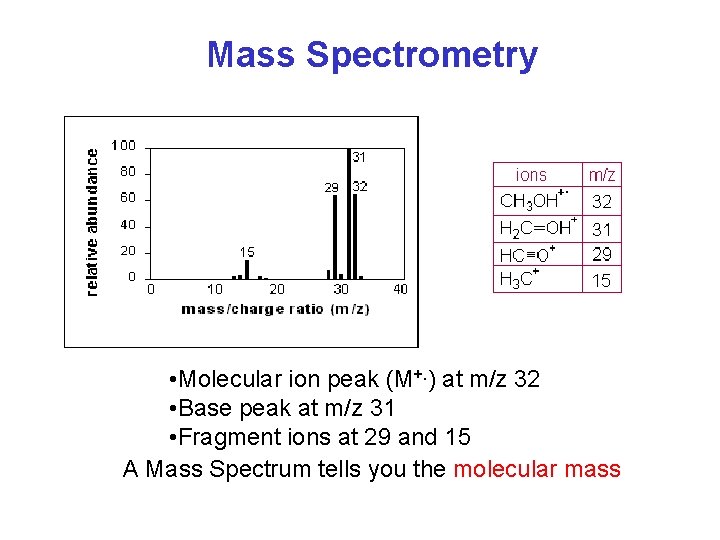
Mass Spectrometry • Molecular ion peak (M+. ) at m/z 32 • Base peak at m/z 31 • Fragment ions at 29 and 15 A Mass Spectrum tells you the molecular mass
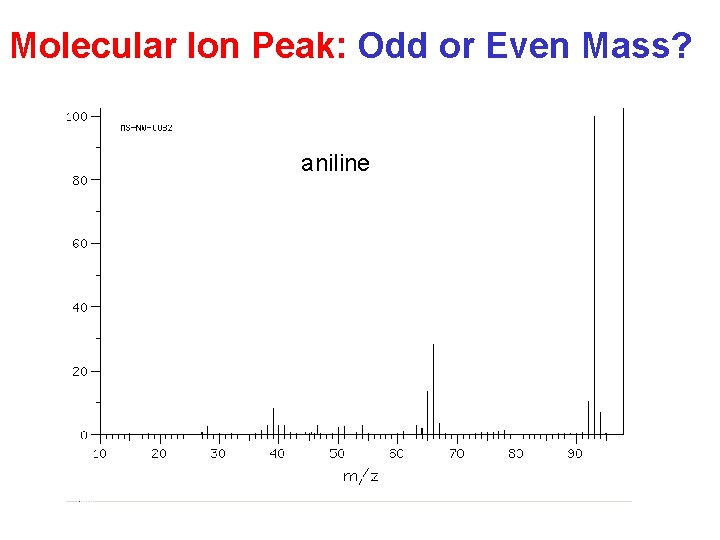
Molecular Ion Peak: Odd or Even Mass? aniline
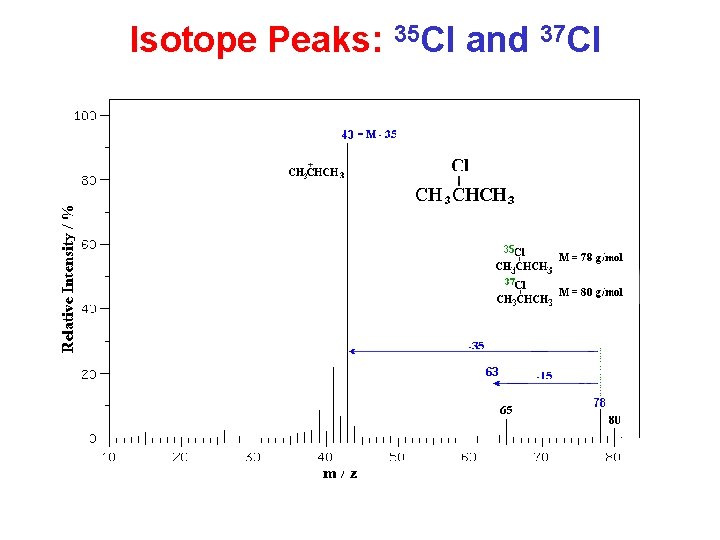
Isotope Peaks: 35 Cl and 37 Cl
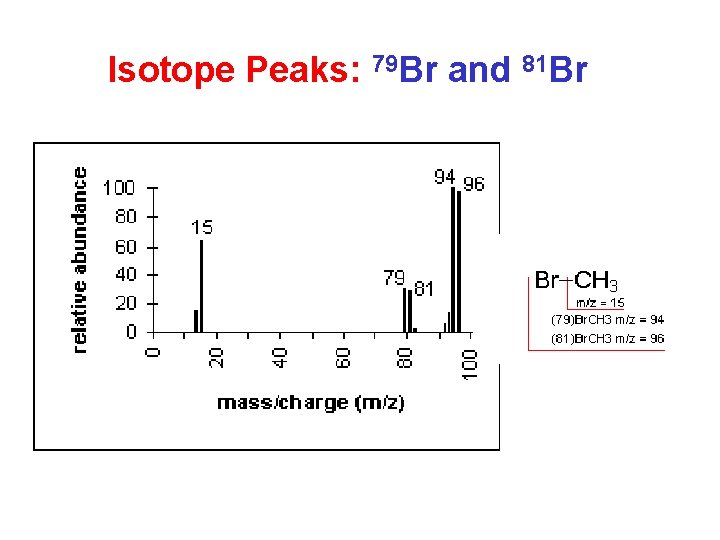
Isotope Peaks: 79 Br and 81 Br
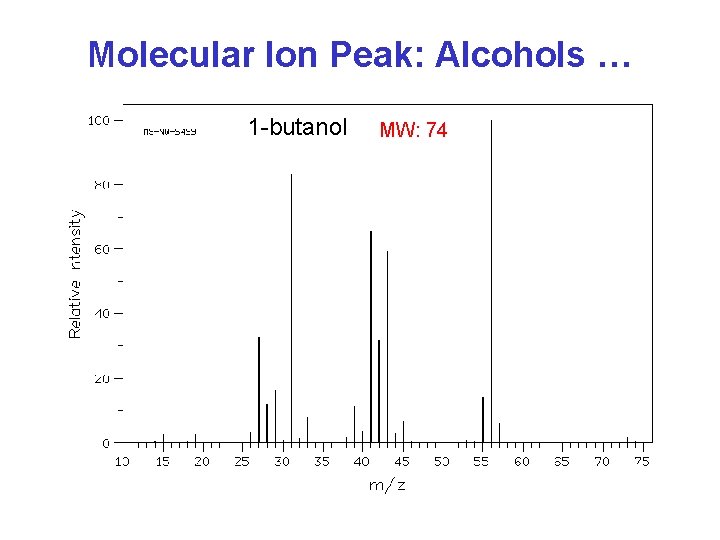
Molecular Ion Peak: Alcohols … 1 -butanol MW: 74
Favored Fragmentations • The greater the stability of the fragment ion, the more intense the peak • Alkyl ion stability follows carbocation chemistry rules: 3° and allyl more stable than 2° 2° more stable than 1° • Acyl ions are stable (m/z 43, 57. . ) – Ph. C=O+ (m/z 105…) • Alpha (a) cleavage in alcohols, amines, ethers • Aromatic rings do NOT fragment easily

Characteristic Fragment Ions • Look for alkyl ions at m/z 43, 57, 71, 85 (14 amu or CH 2 series)
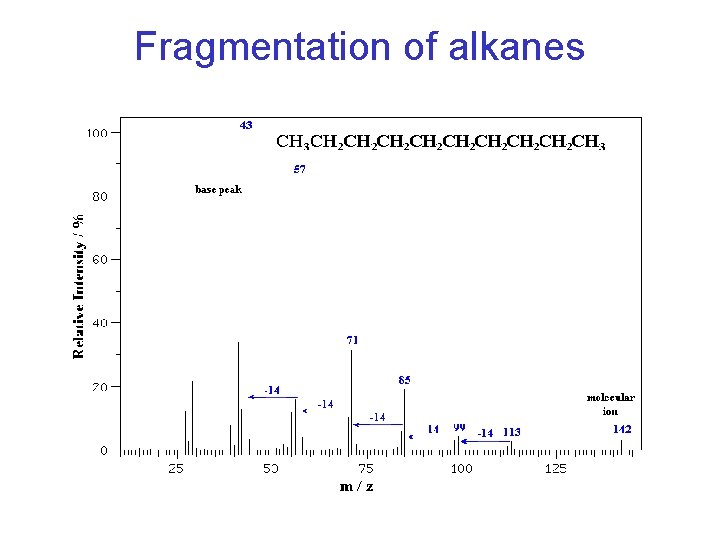
Fragmentation of alkanes

Characteristic Fragment Ions • Look for aryl ions at 77 (phenyl), 91 (benzyl), and 105 (benzoyl)
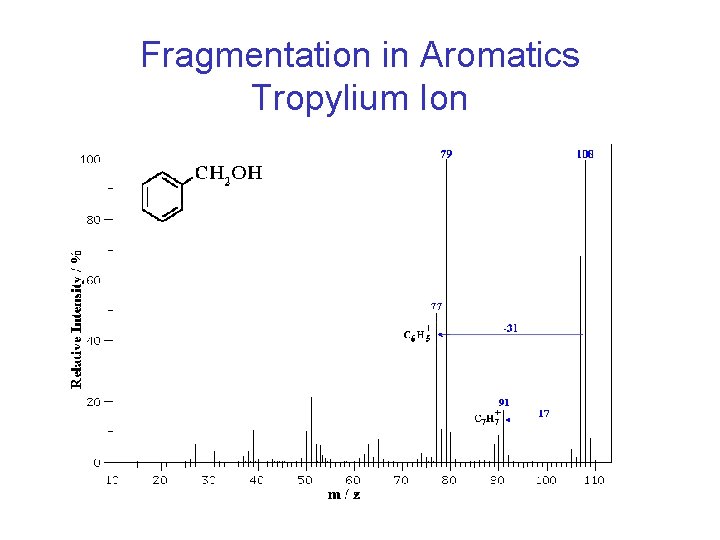
Fragmentation in Aromatics Tropylium Ion

Characteristic Fragment Ions • Acyl ions are stable (m/z 43, 57. . )
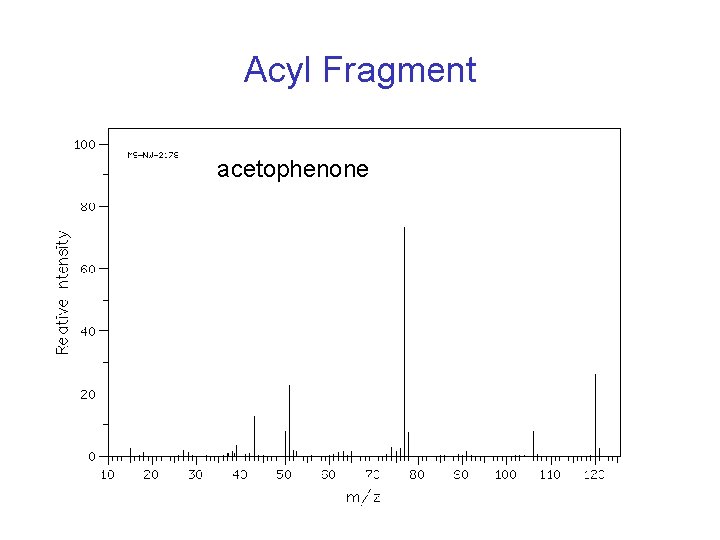
Acyl Fragment acetophenone

Characteristic Neutral Losses • • • 1 is H (e. g. from aldehydes) 15 can only be methyl 17 usually OH 18 always H 2 O 28 CO or CH 2=CH 2 29 CH 3 CH 2 or CHO 31 CH 3 O 35/37 Cl (special isotope pattern too!) 42 CH 2=C=O 43 CH 3 CO or C 3 H 7 45 CH 3 CH 2 O or COOH 79/81 Br (special isotope pattern too!)

Weights of common fragments or neutral losses
Mass Spectrometry Summary Molecular weight: • odd-numbered (N. . . ) ? • isotope peaks (Cl, Br)? • Alcohols … Recognize typical fragments: H 2 O, alkyl, acyl, tropylium ion …

Next time: Bring Spectra of your Unknown #3! • Brief Review of Mass Spectroscopy • Discussion/Review of H-NMR, CMR
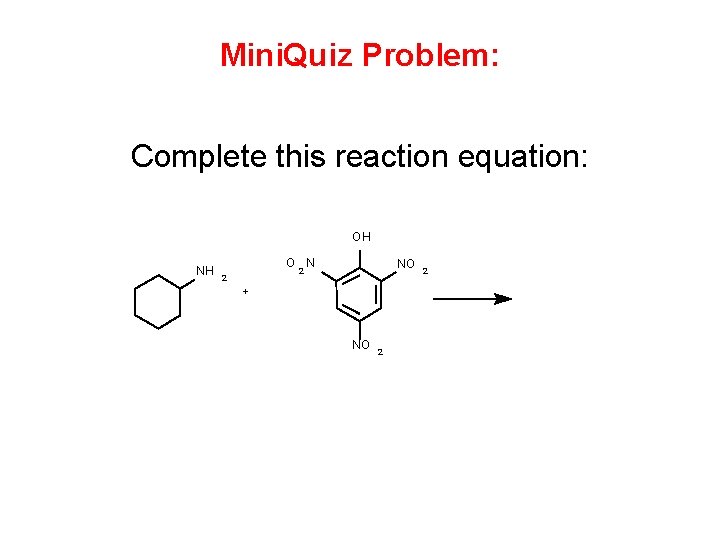
Mini. Quiz Problem: Complete this reaction equation: OH NH O N NO 2 2 + NO 2 2
- Slides: 20