Minerals S 6 E 5 Students will investigate






- Slides: 10

Minerals S 6 E 5 Students will investigate the scientific view of how the earth's surface is formed.

What’s a Mineral? Mineral : n Natural occurring, inorganic n Can be one or a combination of chemicals n About 3, 000 known minerals n Crystal structure (atoms are arranged within the mineral in a specific ordered way) n Homogeneous - same substance chemically throughout

How Minerals Form Minerals can form in two ways: n From hot magma or lava as igneous mineral n From the evaporation of hot water solutions. When minerals form from solutions, the solution evaporates, and leaves the mineral behind.

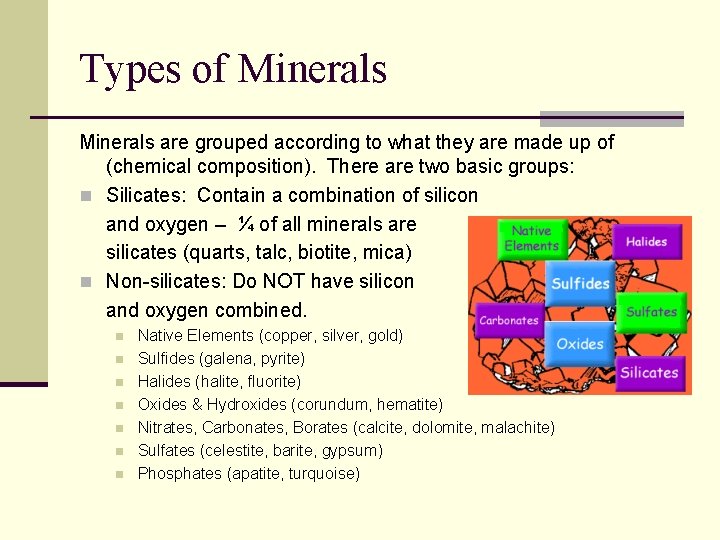
Types of Minerals are grouped according to what they are made up of (chemical composition). There are two basic groups: n Silicates: Contain a combination of silicon and oxygen – ¼ of all minerals are silicates (quarts, talc, biotite, mica) n Non-silicates: Do NOT have silicon and oxygen combined. n n n n Native Elements (copper, silver, gold) Sulfides (galena, pyrite) Halides (halite, fluorite) Oxides & Hydroxides (corundum, hematite) Nitrates, Carbonates, Borates (calcite, dolomite, malachite) Sulfates (celestite, barite, gypsum) Phosphates (apatite, turquoise)

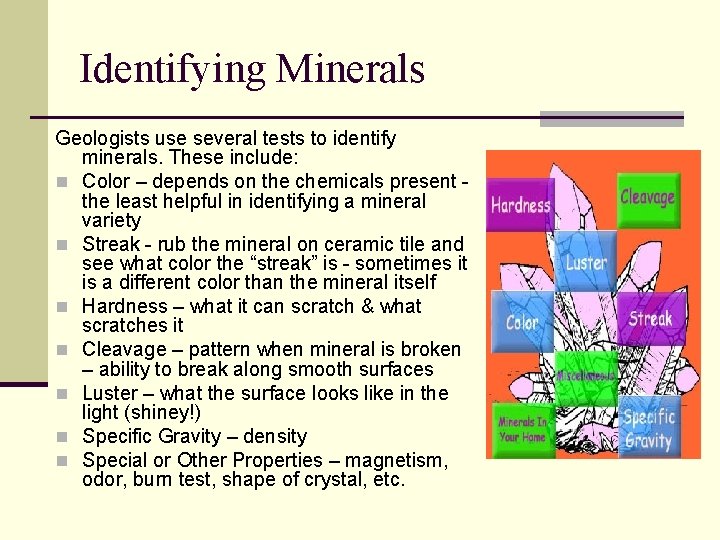
Identifying Minerals Geologists use several tests to identify minerals. These include: n Color – depends on the chemicals present the least helpful in identifying a mineral variety n Streak - rub the mineral on ceramic tile and see what color the “streak” is - sometimes it is a different color than the mineral itself n Hardness – what it can scratch & what scratches it n Cleavage – pattern when mineral is broken – ability to break along smooth surfaces n Luster – what the surface looks like in the light (shiney!) n Specific Gravity – density n Special or Other Properties – magnetism, odor, burn test, shape of crystal, etc.

Color, Streak & Hardness Color: the most obvious characteristic but the least reliable. Some minerals come in different colors Streak: determined when an unknown mineral is rubbed against a piece of unglazed porcelain (streak plate). It may produce a colored line which will help to determine the minerals identification. Hardness: how resistant a mineral is to being scratched. We use the Mohs scale to classify a given minerals hardness, which is a rating system 1 – 10.

Clevage, Luster & Specific Gravity Cleavage: the result of the way the chemicals bond together and the flat surfaces and angles at which they meet. Luster: the way a mineral reflects light. Generally this means is it metallic or non-metalic. Specific Gravity: the minerals density. If the mineral is heavy for its size, then it has a high specific gravity.

Crystals are minerals that have had the chance to grow in the shape that they were meant to be. The chemicals that a mineral is made of determines what shape it gets to be. We can tell different minerals apart by what crystal shape they are. To form a crystal shape minerals need specific conditions: n LOTS of time (they form slowly) n Room to “grow” When minerals cool slow enough their atoms have time to arrange in flat surfaces with regular angles. Sometimes crystals form when liquids underground find their way into cracks and slowly deposit minerals.

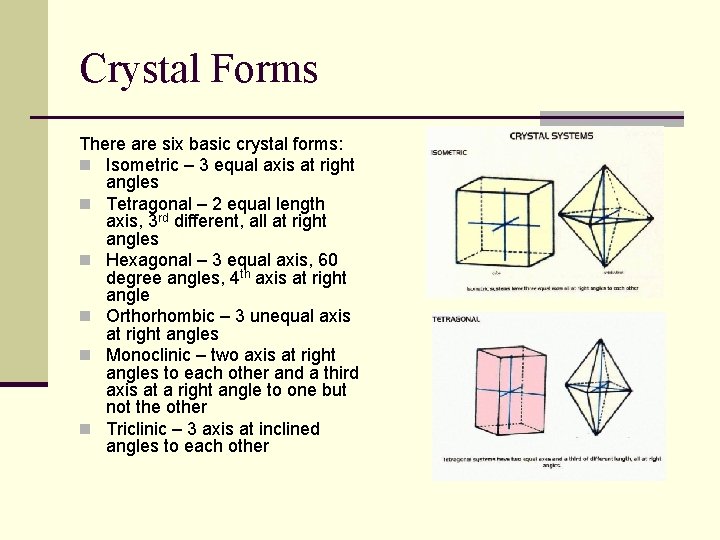
Crystal Forms There are six basic crystal forms: n Isometric – 3 equal axis at right angles n Tetragonal – 2 equal length axis, 3 rd different, all at right angles n Hexagonal – 3 equal axis, 60 degree angles, 4 th axis at right angle n Orthorhombic – 3 unequal axis at right angles n Monoclinic – two axis at right angles to each other and a third axis at a right angle to one but not the other n Triclinic – 3 axis at inclined angles to each other

Activities! n Minerals Worksheet n Minerals Online Scavenger Hunt Worksheet