Minerals Layers of the Earth Crust 8 to




































- Slides: 39


Minerals

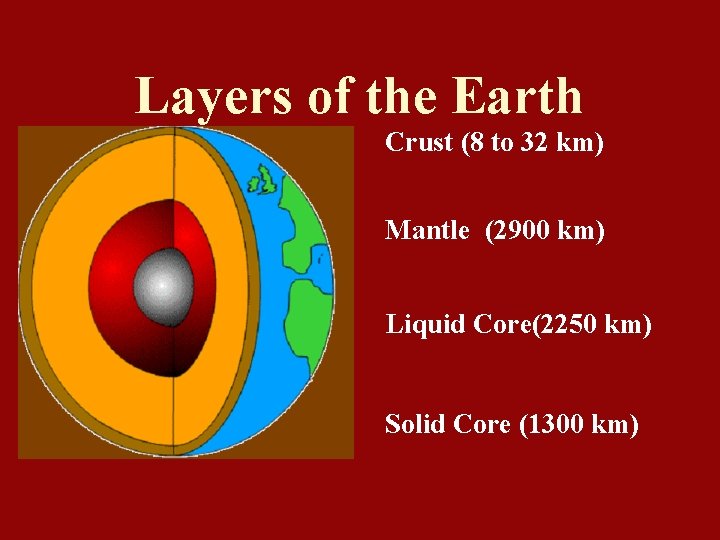
Layers of the Earth Crust (8 to 32 km) Mantle (2900 km) Liquid Core(2250 km) Solid Core (1300 km)

Core 1. Solid Inner Core: • Made of solid Nickel and Iron • Under great pressure resulting in a solid form. • Iron causes the Earth to be magnetic • 5000 degrees Celsius

2. Liquid Outer Core • • Made of liquid Nickel and Iron 2200 degrees Celsius

Mantle • • Molten, liquid rock (Magma) Makes up about 80% of Earth’s volume Ranges from 870 to 2200 degrees Celsius Crust and Mantle divided by the Moho Boundary

Crust • • Very thin, solid layer of rock Made of mostly Silicon & Oxygen (75%)

What are Minerals? • • • Naturally occurring – not man-made, in the earth Inorganic – doesn’t come from living things Always a solid – has volume and shape

• • Definite chemical composition – made of a single pure substance or element Crystalline form – flat sides, sharp edges & corners

Formation & Composition • • As hot magma cools, the minerals will crystallize. If the magma cools very slowly it forms large crystals. If it cools very quickly it forms tiny to microscopic crystals.

• Some mineral crystals will form compounds dissolved in liquids. Minerals will be left behind when the liquid evaporates.

• The eight most common elements in the crust form a large number of the known minerals. (O, Si, Al, Fe, Ca, Na, K, & Mg) • The compound formed by Silicon & Oxygen is known as Silicate.

Mineral Identification • The identification of a mineral is based on its physical properties

1. Color • Some minerals have characteristic colors • Some minerals can come in several different color varieties

• EX: Quartz & Corundum • Some of the colors can change due to temperature, pollution, or radiation.

2. Luster • • The way a mineral reflects light off its surface. 2 types

A. Metallic – very shiny, looks like a metal

B. Non-metallic • Glassy – shiny, transparent, translucent

• Earthy - clay-like, dull • Pearly: pearl-like

3. Hardness • The ability for a mineral to resist being scratched.

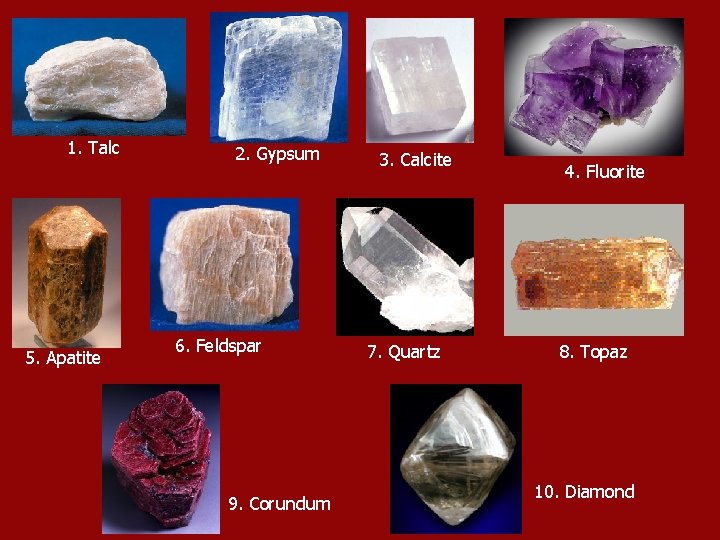
Mohs Hardness Scale • A list of ten minerals ranked according to hardness. 1= softest (talc) 10=hardest (diamond)

1. Talc 5. Apatite 2. Gypsum 6. Feldspar 9. Corundum 3. Calcite 7. Quartz 4. Fluorite 8. Topaz 10. Diamond

• A mineral that is higher on the scale will scratch the mineral that is lower. Other common items have been given ratings on the scale for identification purposes. • EX: Fingernail, Penny, File, Glass •

4. Streak • • • The color of the powder scraped off a mineral when rubbed against a rough surface (streak plate. ) Very useful property for showing the TRUE color. Same color always, no matter what the variation


5. Breakage 2 types – a. Cleavage - breaks in smooth, definite surfaces • Same every time

b. Fracture - breaks in rough jagged surfaces or

6. Crystal Form • Geometric crystals with flat surfaces and definite edges • Crystals can grow very large if there is space and time to cool slowly. • Different from cleavage

7. Density • • each mineral has its own specific density = mass volume

8. Special Properties 1. 2. Reaction to Acid some minerals will bubble in acid Magnetism some special minerals will be magnetic

3. Smell some will have distinct odors Sulfur 4. Taste some have a distinct taste Halite

Uses for Minerals 1. Ores Minerals or combinations of minerals from which metals and nonmetals can be removed in usable amounts.

a. Metals • Elements that have shiny surfaces • • Conducts electricity & heat Can be easily shaped breaking Ex: Fe, Pb, Al, Cu, Ag, & Au without

• Smelting- when an ore is heated so that a metal can be separated from it. • Alloys- when pure metals are combined form other metallic substances. (Steel & Brass)

b. Nonmetals • Elements that have dull surfaces • Poor conductors of electricity & heat • Not easily shaped Ex: S & F

2. Gemstones Minerals that hard, beautiful, and durable substances that can be cut & polished for jewelry & decoration.

a. Precious Stones • rarest & most valuable Ex: Diamonds, Rubies, Opals, Sapphires, & Emeralds

b. Semiprecious Stones • all other gemstones Ex: Amethysts, Zircons, Garnets, Turquoises, Jades, & more

c. Non-Mineral Gemstones • came from living things Ex: Amber & Pearls