Minerals Building Blocks of Rocks and Economic Resources

Minerals Building Blocks of Rocks and Economic Resources GLY 2010 - Summer 2012 Lecture 4 1

Minerals • Minerals are a major building block of most rocks • Their properties determine a good part of the physical behavior of the earth 2

Mineral Definition • Naturally occurring • Inorganic • Crystalline 3

Crystal Structure Examples Halite Fluorite 4
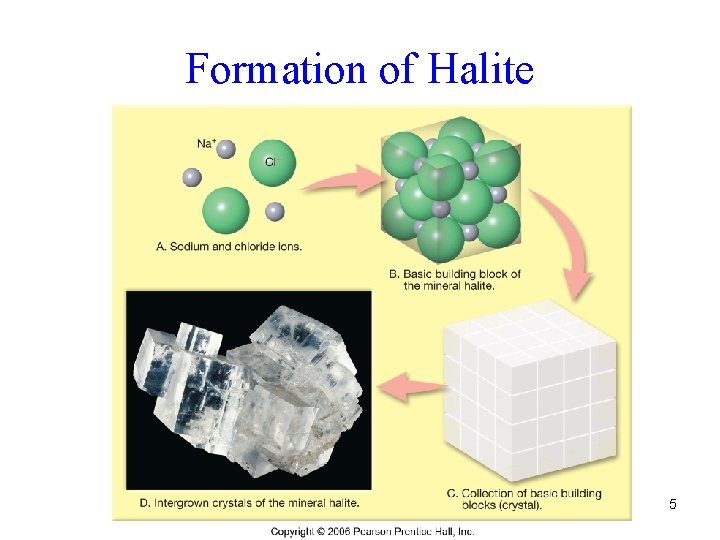
Formation of Halite 5

Atoms 6
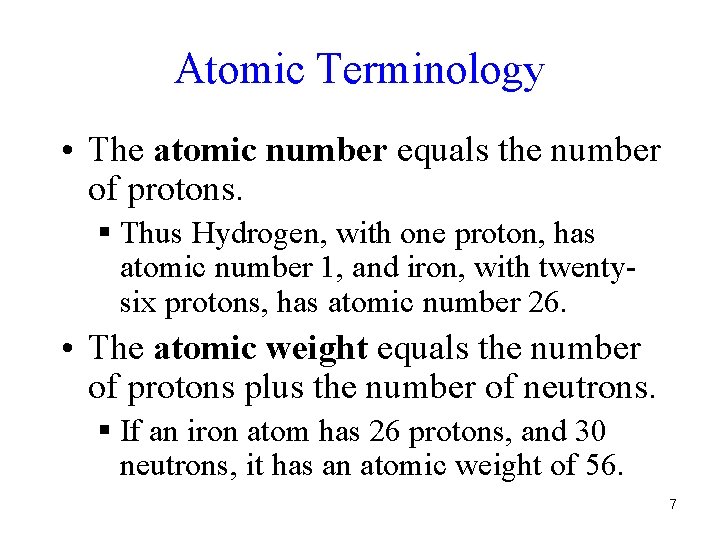
Atomic Terminology • The atomic number equals the number of protons. § Thus Hydrogen, with one proton, has atomic number 1, and iron, with twentysix protons, has atomic number 26. • The atomic weight equals the number of protons plus the number of neutrons. § If an iron atom has 26 protons, and 30 neutrons, it has an atomic weight of 56. 7
Chemical Elements • An element is composed of atoms with the same atomic number • Each element has a unique chemical symbol 8

Isotopes • An isotope of an element is an atom with the correct number of protons for that element, plus a fixed number of neutrons § Example: Carbon has three isotopes, each with six protons, and with 6, 7, or 8 neutrons 9
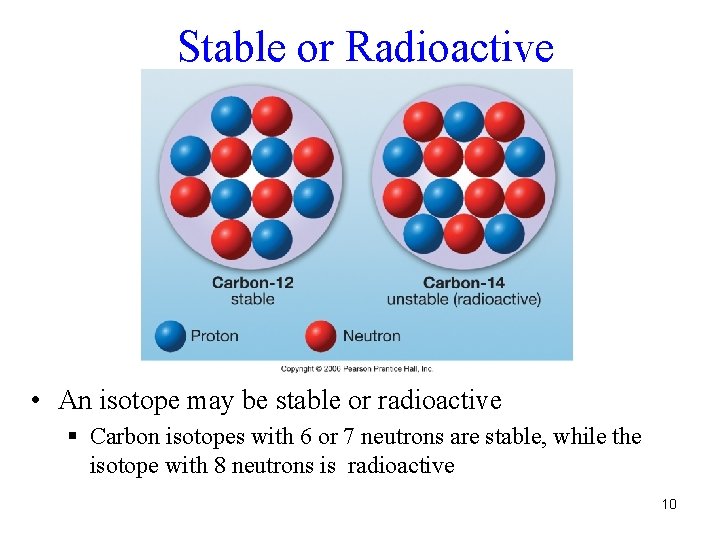
Stable or Radioactive • An isotope may be stable or radioactive § Carbon isotopes with 6 or 7 neutrons are stable, while the isotope with 8 neutrons is radioactive 10
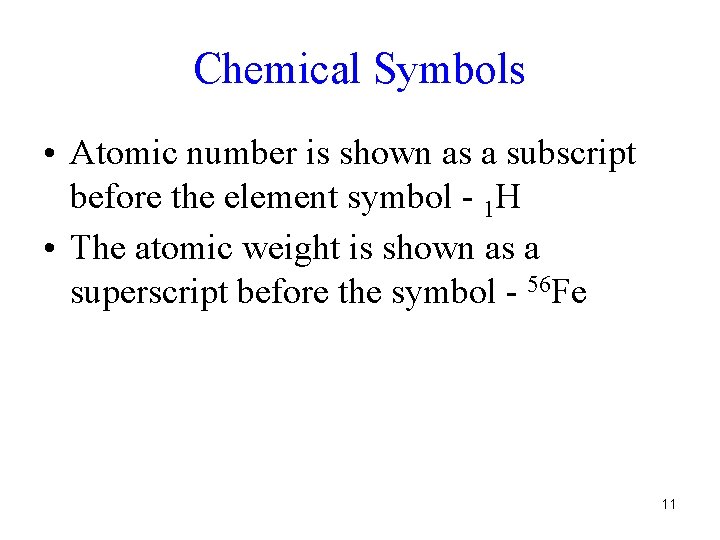
Chemical Symbols • Atomic number is shown as a subscript before the element symbol - 1 H • The atomic weight is shown as a superscript before the symbol - 56 Fe 11
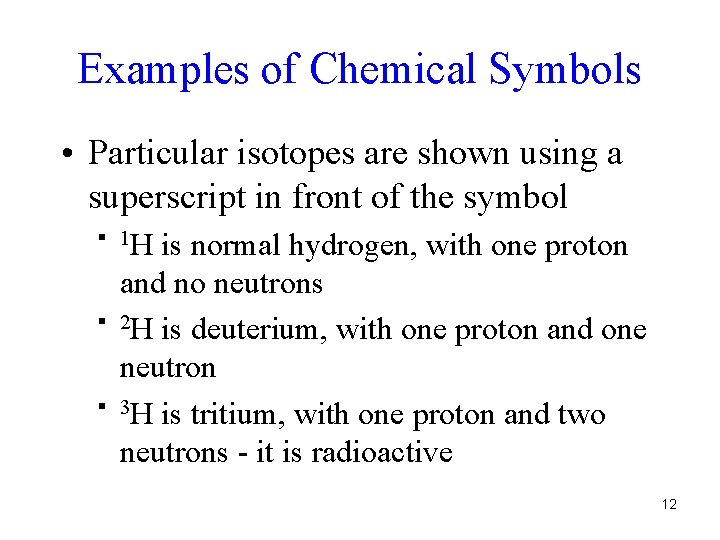
Examples of Chemical Symbols • Particular isotopes are shown using a superscript in front of the symbol § 1 H § § is normal hydrogen, with one proton and no neutrons 2 H is deuterium, with one proton and one neutron 3 H is tritium, with one proton and two neutrons - it is radioactive 12
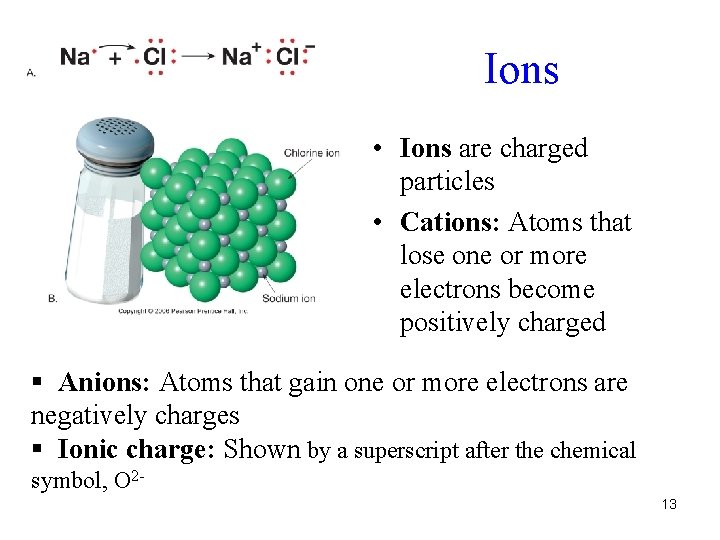
Ions • Ions are charged particles • Cations: Atoms that lose one or more electrons become positively charged § Anions: Atoms that gain one or more electrons are negatively charges § Ionic charge: Shown by a superscript after the chemical symbol, O 213

Use of Isotopes • Chemical tracers § Study topics such as: • Pollution • Formation temperature • The path of volcanic emissions, etc • Radioactive isotopes are used in estimating the age of materials 14

Compounds • Combination of two or more atoms • Combination is called a molecule § Water H 2 O § Carbon dioxide CO 2 15
Molecules • Molecules may consist of just one element § Oxygen in the atmosphere is O 2 • Molecules may consist of several elements, in various amounts § Example: Plagioclase feldspar, the most common mineral on earth Na. Al. Si 3 O 8 - one sodium (Na), one aluminum (Al), three silicons (Si), and eight oxygens (O) 16

Chemical Bonds • The “glue” that holds materials together • Responsible for the properties of matter § On an atomic scale § At the scale of the earth • When two atoms combine to form a chemical bond, energy is released 17

Types of Bonds • • • Ionic Covalent Metallic Hydrogen Van der Waals 18
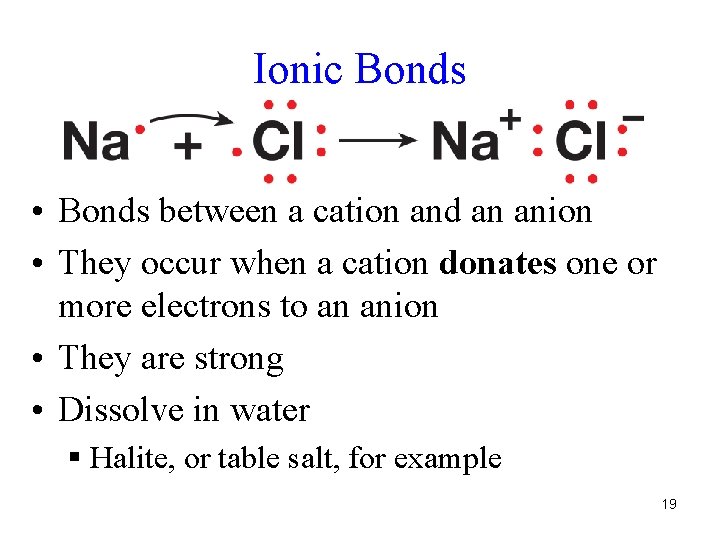
Ionic Bonds • Bonds between a cation and an anion • They occur when a cation donates one or more electrons to an anion • They are strong • Dissolve in water § Halite, or table salt, for example 19
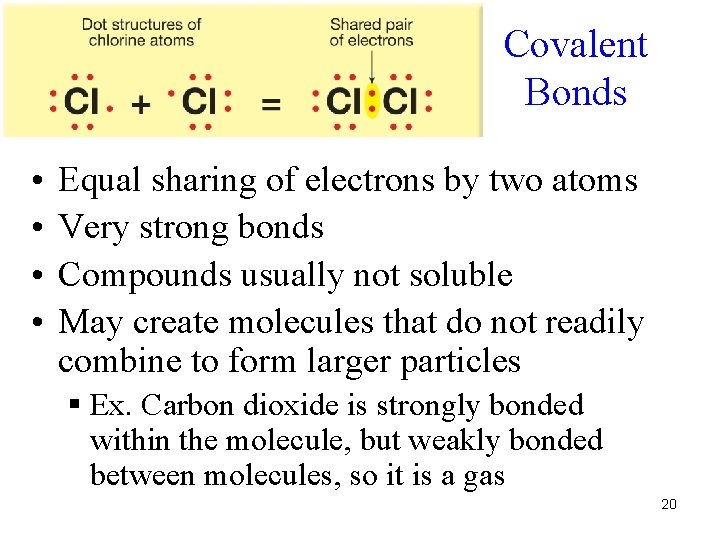
Covalent Bonds • • Equal sharing of electrons by two atoms Very strong bonds Compounds usually not soluble May create molecules that do not readily combine to form larger particles § Ex. Carbon dioxide is strongly bonded within the molecule, but weakly bonded between molecules, so it is a gas 20

Metallic Bonds • Outer electrons are loosely held • Properties: Opaque, may have a metallic luster • Bond strength is moderate 21
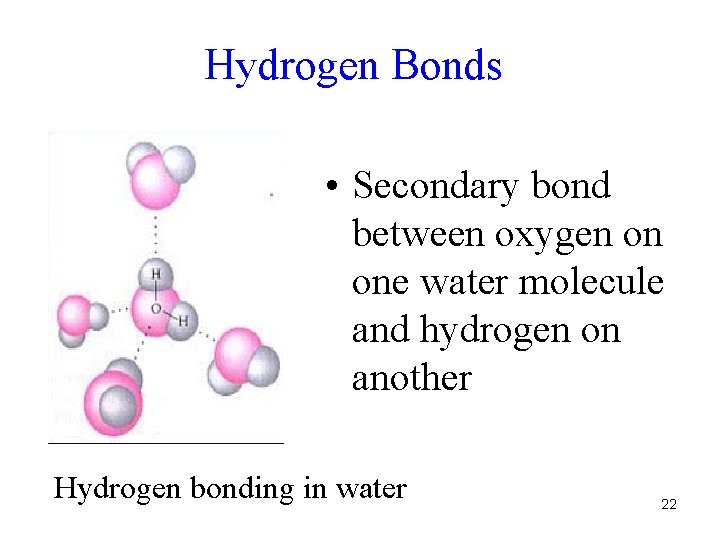
Hydrogen Bonds • Secondary bond between oxygen on one water molecule and hydrogen on another Hydrogen bonding in water 22
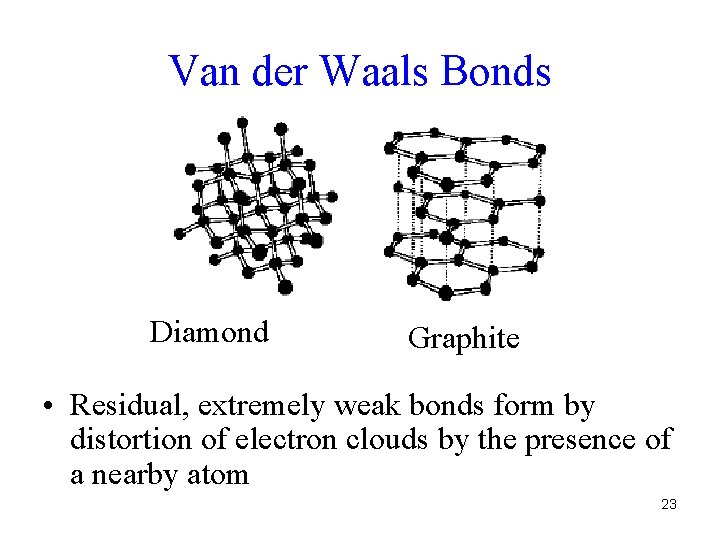
Van der Waals Bonds Diamond Graphite • Residual, extremely weak bonds form by distortion of electron clouds by the presence of a nearby atom 23
Molecular Properties • Molecules - as strong as the weakest bonds within themselves 24

Mineral Properties • Depend on the type and strength of bonds and number of bonds (bond density) within themselves • Minerals will be examined in the laboratory, and most properties will be taught there • Examples of mineral properties: hardness, cleavage 25

Hardness • A mineral’s hardness is measured by the ability of a surface to resist abrasion 26
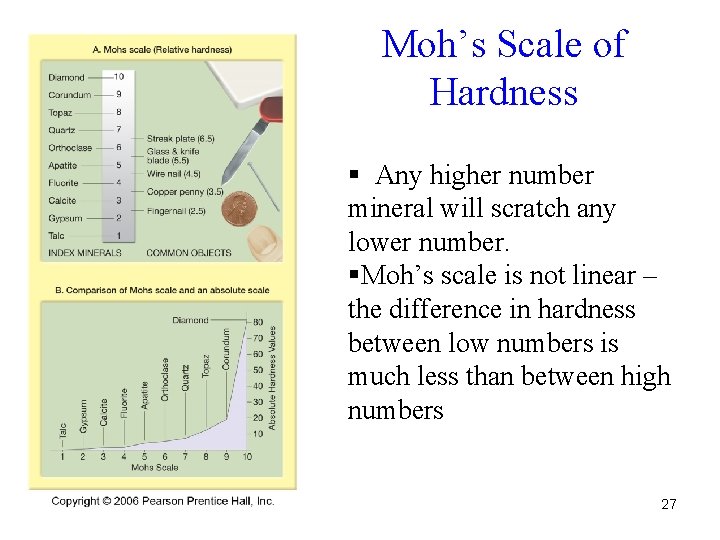
Moh’s Scale of Hardness § Any higher number mineral will scratch any lower number. §Moh’s scale is not linear – the difference in hardness between low numbers is much less than between high numbers 27

Cleavage • When a mineral always or usually breaks along a particular plane, it is said to have a cleavage plane 28

Two-directional Cleavage • Selenite, a variety of the mineral gypsum, shows cleavage in two directions 29

Angle Between Cleavage Planes 30

Three-directional Cleavage • Halite, common table salt, shows three directions of cleavage at right angles 31

Three-directional Cleavage • Calcite shows three directions of cleavage, not at right angles 32
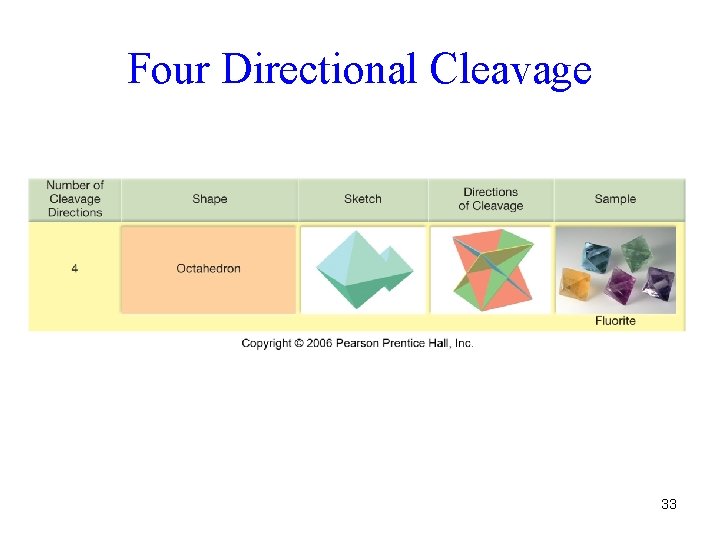
Four Directional Cleavage 33

Crystal and Crystal Faces Apatite, showing a hexagonal prism these are crystal faces, not cleavage planes 34

Identification of Minerals • Minerals are identified based on their physical and chemical properties • A combination of properties are needed, just as no single line from a fingerprint can identify a person 35

Mineral Classification • Classification is based on anion type § Minerals with the same anions have similar properties, while those with the same cations often do not 36

Anions • Anions may be a single ion § Ex. Oxygen O 2 - • Anions are often groups of atoms, with the entire group having a negative charge § Ex. Carbonates are CO 32 - , one carbon with three oxygens, and the whole group with a minus 2 charge 37

Common Anion Groups • • • Silicates, Si. O 44 Oxides, O 2 Sulfides, S 2 Carbonates, CO 32 Phosphates, PO 4338

Occurrence of Minerals • Over half of all known minerals are silicates, because oxygen is the most common element on earth, and silicon is the second most common. • Silicates are the most important type of rockforming minerals, those minerals that make up most of the earth’s rocks • Most silicate minerals contain other elements in addition to silicon and oxygen 39

Silicon Tetrahedron • The Si. O 44 tetrahedron is the basic building block of silicate minerals 40

Silicate Structures 41
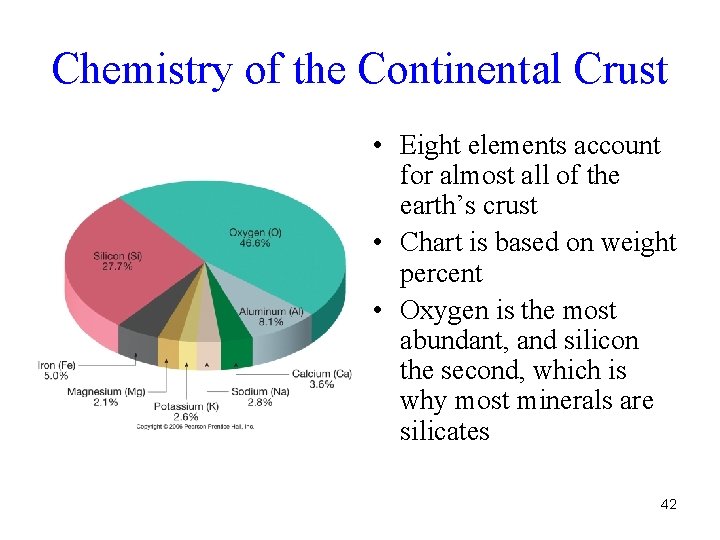
Chemistry of the Continental Crust • Eight elements account for almost all of the earth’s crust • Chart is based on weight percent • Oxygen is the most abundant, and silicon the second, which is why most minerals are silicates 42

Felsic Minerals • Minerals with a lot of aluminum and silicon are light in color, and are called Felsic • Plagioclase feldspar, the most common mineral in the earth’s crust 43

Mafic Minerals • Minerals with more iron and magnesium, and less silicon, are dark in color and are called Mafic (from the first two letters of magnesium and the first letter of ferium) • Augite, a type of pyroxene 44
- Slides: 44