Mineralocorticoid Receptor activation in Perivascular adipose tissue macrophages









- Slides: 27

Mineralocorticoid Receptor activation in Perivascular adipose tissue macrophages and diet induced vascular stiffness Guido Lastra MD University of Missouri Division of Endocrinology and Diabetes Harry S. Truman VA Hospital Columbia, Missouri

Disclosures and conflicts of interest Nothing to disclose

Adipose tissue and cardiovascular disease • Cardiovascular disease (CVD) risk related to obesity is more strongly associated with visceral rather than subcutaneous adiposity • Limited sensitivy and specificity of anthropometric measurements • Newer techniques (MRI, CT scan, DXA) allow for study of additional adipose tissue deposits – Visceral – PVAT

Adipose tissue and inflammation • Inflammation in adipose tissue is not a novel finding • Excess caloric intake + reduced energy expenditure • Adipose tissue hyperplasia & hypertrophy • Release of chemotactic factors (MCP-1, etc) • Migration of monocytes into adipose tissue and macrophage polarization/infiltration (classic pathway of M 1)

Adipose tissue and inflammation • Consequences: – Adipocyte apoptosis, increased lipolysis, local free fatty acids, reactive oxygen species (ROS), tissue hypoxia – Local production of cytokines such as interleukin (IL)-1 b, TNF-a, and IL-6 – Insulin resistance

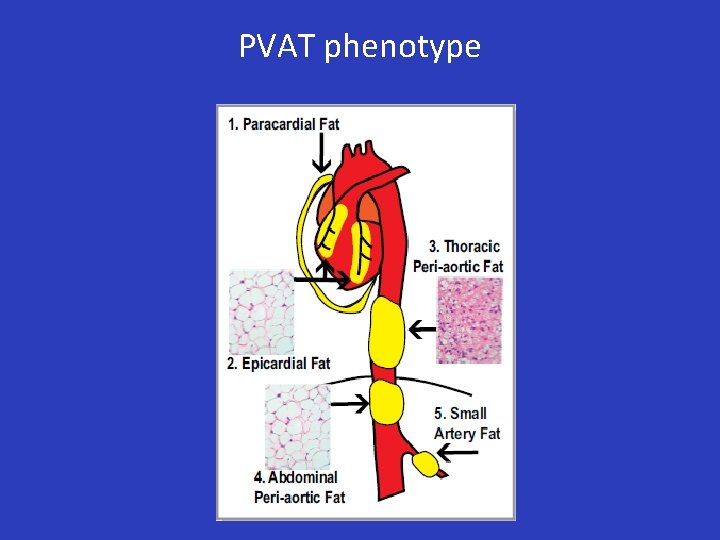
Perivascular Adipose Tissue: PVAT • Perivascular and epicardial fat are normally present in humans • PVAT surrounds large (aorta), medium-size (mesenteric), and small arteries (gluteal) • Obesity: increase in the size of PVAT, proportional to visceral fat

PVAT phenotype

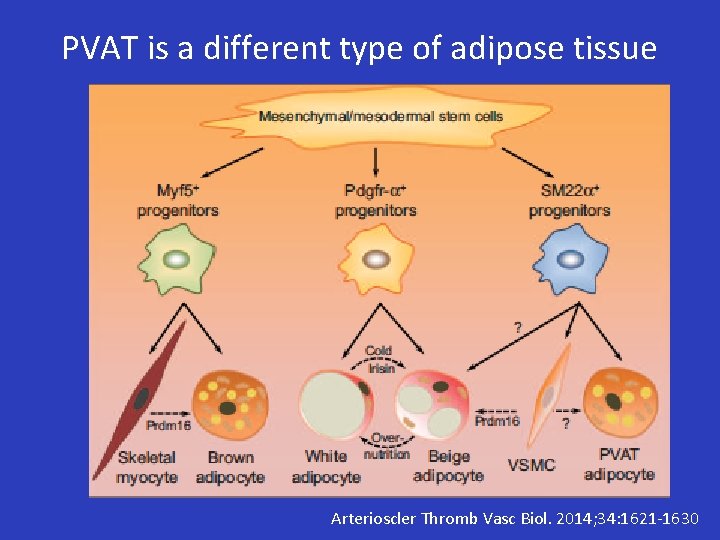
PVAT is a different type of adipose tissue Arterioscler Thromb Vasc Biol. 2014; 34: 1621 -1630

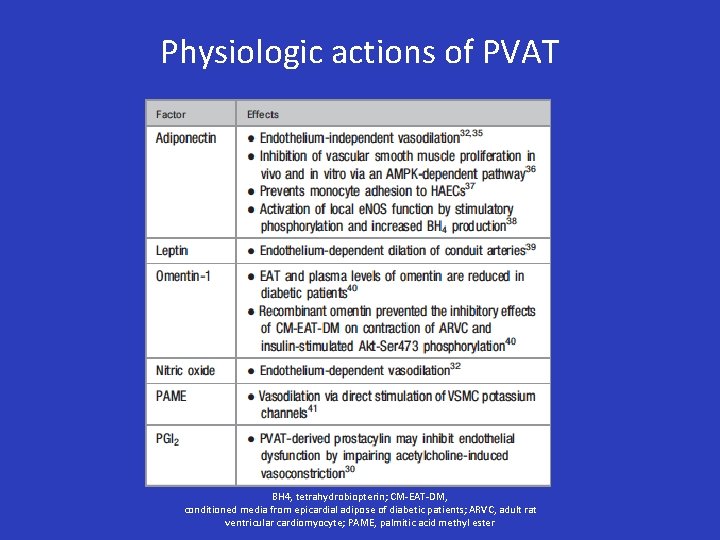
Physiologic actions of PVAT BH 4, tetrahydrobiopterin; CM-EAT-DM, conditioned media from epicardial adipose of diabetic patients; ARVC, adult rat ventricular cardiomyocyte; PAME, palmitic acid methyl ester

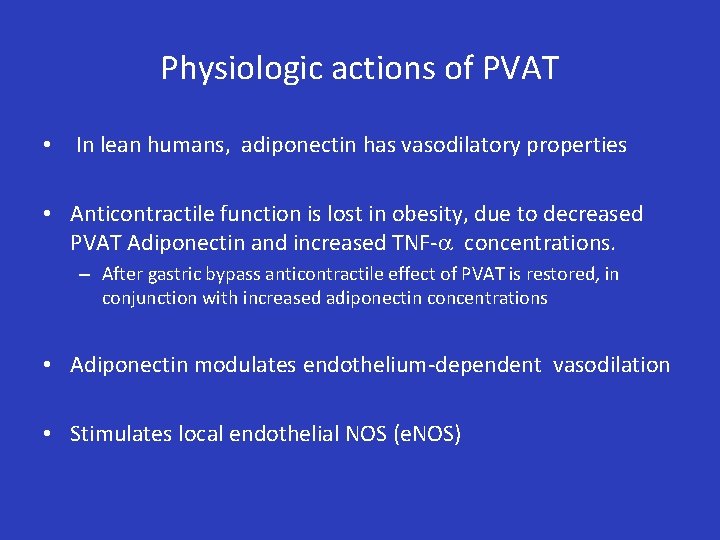
Physiologic actions of PVAT • In lean humans, adiponectin has vasodilatory properties • Anticontractile function is lost in obesity, due to decreased PVAT Adiponectin and increased TNF-a concentrations. – After gastric bypass anticontractile effect of PVAT is restored, in conjunction with increased adiponectin concentrations • Adiponectin modulates endothelium-dependent vasodilation • Stimulates local endothelial NOS (e. NOS)

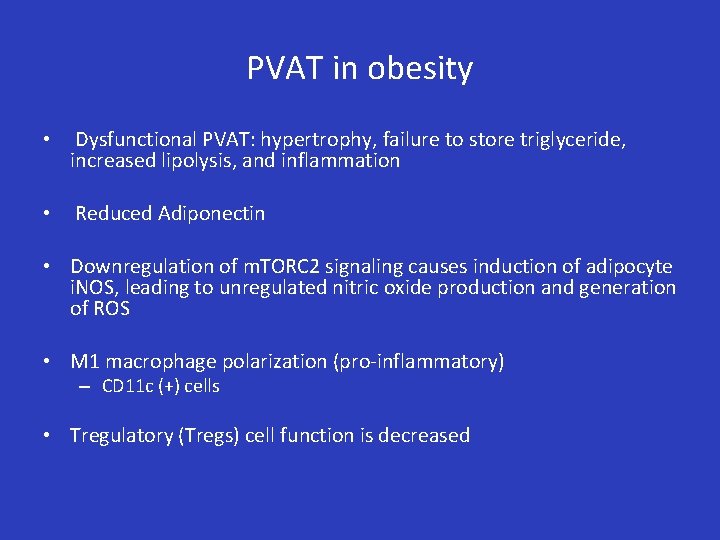
PVAT in obesity • Dysfunctional PVAT: hypertrophy, failure to store triglyceride, increased lipolysis, and inflammation • Reduced Adiponectin • Downregulation of m. TORC 2 signaling causes induction of adipocyte i. NOS, leading to unregulated nitric oxide production and generation of ROS • M 1 macrophage polarization (pro-inflammatory) – CD 11 c (+) cells • Tregulatory (Tregs) cell function is decreased

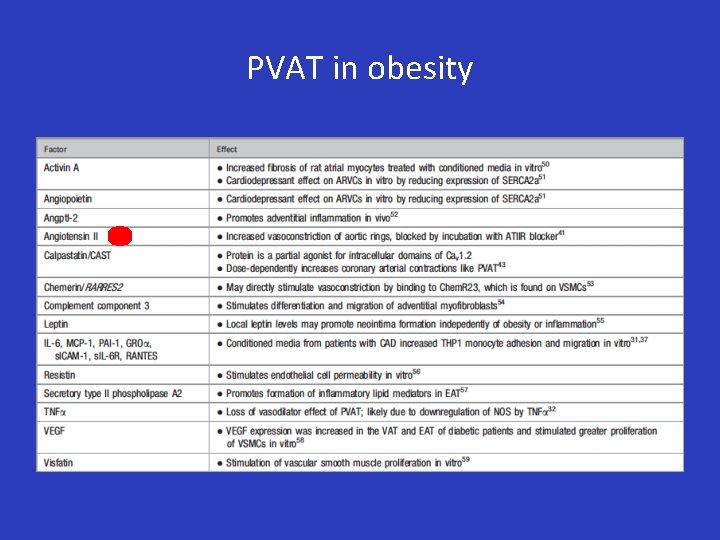
PVAT in obesity

PVAT in obesity • RAAS activation: Mineralocorticoid Receptor (MR) – Aldosterone is produced by adipocytes and MR blockade only improves endothelial-dependent relaxation in the presence of PVAT (Briones AM, Hypertension. 2012; 59(5): 1069 -78 ) – Role of MR activation on loss of Tregs function and M 1/M 2 macrophage polarization is still unclear

Macrophage polarization • Control of macrophage polarization – Loss of Treg function results in inflammatory M 1 macrophage polarization at the expense of M 2 response – MR is expressed in macrophages • MR KO in macrophages favors M 2 polarization in PVAT and protects against cardiac hypertrophy, fibrosis and vascular remodeling – Usher MG, et al. The Journal of Clinical Investigation. 2010; 120(9): 3350 -64 • Mechanisms by which MR activation in macrophages is regulated, and link to vascular disease are not well understood

Insulin resistance and vascular stiffness • High fat/high fructose diet (Western Diet, WD) leads to insulin resistance • Insulin resistance plays a critical role in the development of CVD • Insulin resistance abrogates vascular protection normally seen in premenopausal women. • Vascular stiffness is strongly correlated with stroke, diastolic heart failure and chronic kidney disease

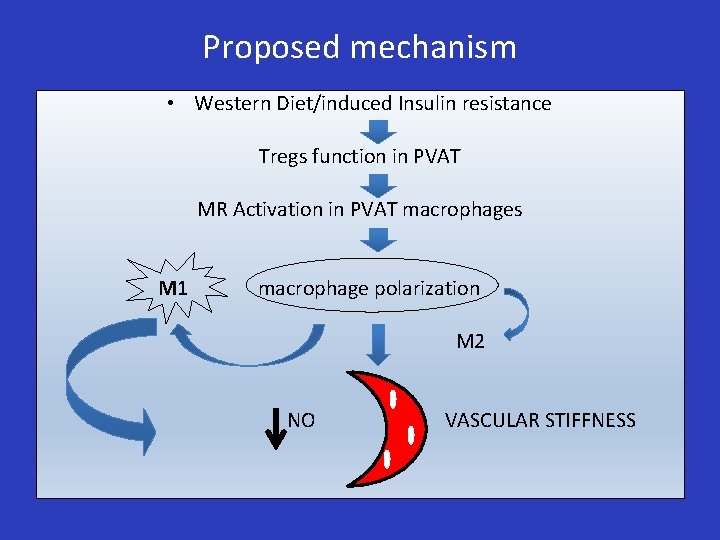
Proposed mechanism • Western Diet/induced Insulin resistance Tregs function in PVAT MR Activation in PVAT macrophages M 1 macrophage polarization M 2 NO VASCULAR STIFFNESS

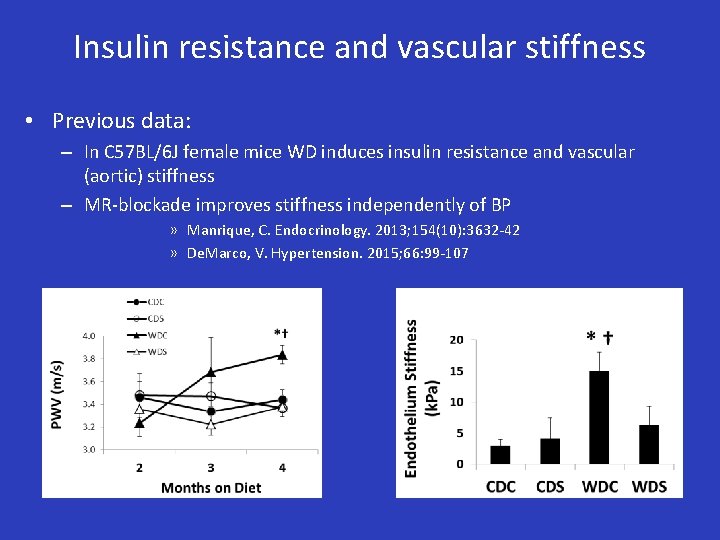
Insulin resistance and vascular stiffness • Previous data: – In C 57 BL/6 J female mice WD induces insulin resistance and vascular (aortic) stiffness – MR-blockade improves stiffness independently of BP » Manrique, C. Endocrinology. 2013; 154(10): 3632 -42 » De. Marco, V. Hypertension. 2015; 66: 99 -107

Aim of the study To determine if lack of MR signaling in macrophages protects against WD and Aldo induced vascular stiffness in female mice

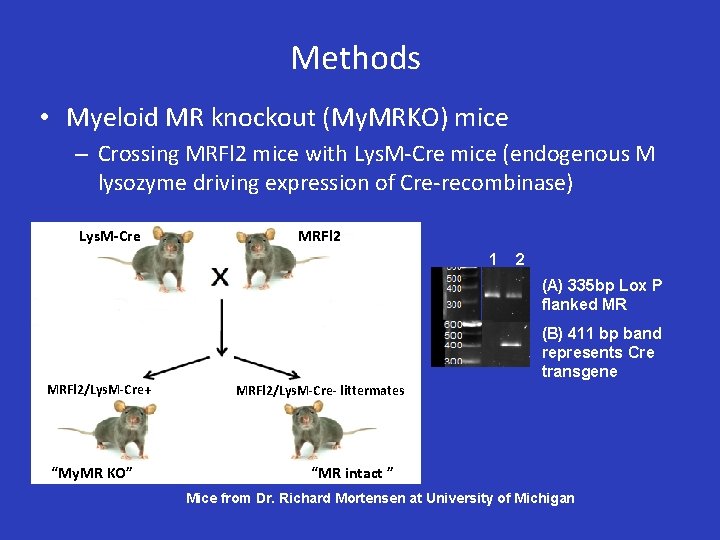
Methods • Myeloid MR knockout (My. MRKO) mice – Crossing MRFl 2 mice with Lys. M-Cre mice (endogenous M lysozyme driving expression of Cre-recombinase) Lys. M-Cre MRFl 2 1 2 (A) 335 bp Lox P flanked MR MRFl 2/Lys. M-Cre+ “My. MR KO” (B) 411 bp band represents Cre transgene MRFl 2/Lys. M-Cre- littermates “MR intact ” Mice from Dr. Richard Mortensen at University of Michigan

Methods • 4 weeks old My. MRKO were fed a WD: 46% fat and 36% carbohydrate (17. 5% each from sucrose and high-fructose corn syrup) for 8 weeks • My. MRKO female mice (18 weeks old) were continuously infused with Aldo (slow pressor dose, 250 mcg/kg/day) for 3 weeks

Methods • Aortic stiffness was assessed in vivo by pulse wave velocity (PWV). • Ex vivo aortic stiffness was measured by atomic force microscopy (AFM) • Vasomotor responses were studied in aortic rings • Fibrosis and macrophage infiltration in PVAT were evaluated in Aldo infused mice by immunohistochemistry (IHC).

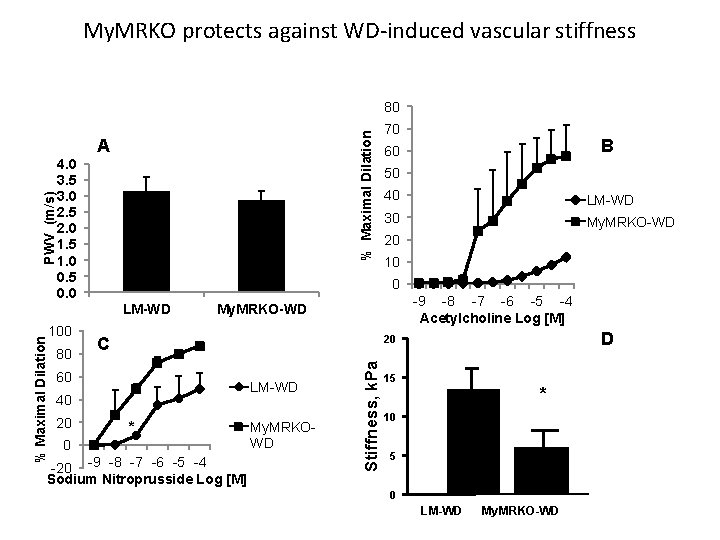
My. MRKO protects against WD-induced vascular stiffness % Maximal Dilation 80 4. 0 3. 5 3. 0 2. 5 2. 0 1. 5 1. 0 0. 5 0. 0 % Maximal Dilation LM-WD 100 80 B 60 50 40 LM-WD 30 My. MRKO-WD 20 10 0 -9 -8 -7 -6 -5 -4 Acetylcholine Log [M] My. MRKO-WD 60 40 20 0 -20 -9 -8 -7 -6 -5 -4 Sodium Nitroprusside Log [M] D 20 C LM-WD My. MRKOWD Stiffness, k. Pa PWV (m/s) A 70 15 * 10 5 0 LM-WD My. MRKO-WD

My. MRKO protects against Aldo-induced vascular stiffness 60 % Maximal dilation A 45 40 35 30 25 20 15 10 5 0 -5 § § § -9 50 LM-CD 40 LM-Aldo 30 § * * -8 -7 -6 Acetylcholine Log [M] * * B 20 My. MRKO + Aldo 10 * 0 -5 -10 -9 -8 * -7 -6 -5 Sodium Nitroprusside Log [M] C § * LM-Aldo My. MRKO+Aldo -4

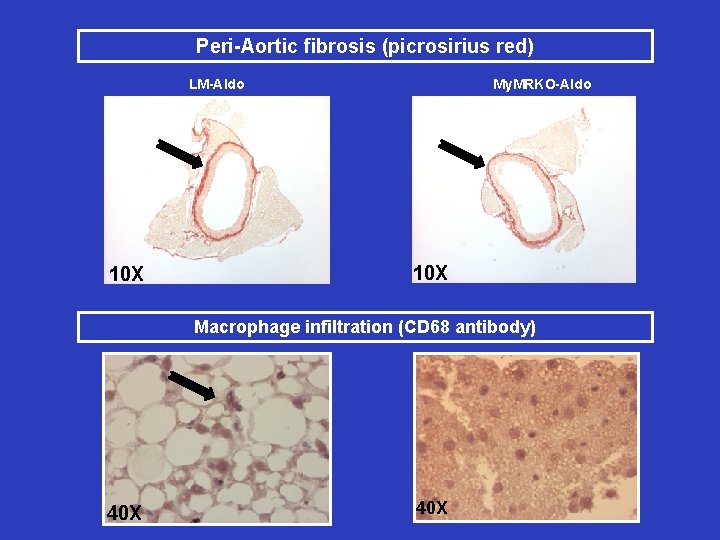
Peri-Aortic fibrosis (picrosirius red) My. MRKO-Aldo LM-Aldo 10 X Macrophage infiltration (CD 68 antibody) My. MRKO+Aldo 40 X

Results: Summary • WD feeding paradigm – WD-fed My. MRKO mice had reduced aortic stiffness measured by AFM – My. MRKO mice fed a WD had improved vasodilatory response • Aldo infusion paradigm – My. MRKO showed decreased aortic stiffness assessed by AFM – Endothelial-dependent and independent dilation were greater in the My. MRKO – IHC in abdominal PVAT was suggestive of decreased fibrosis and macrophage infiltration in Aldo My. MRKO mice

Conclusions • In female mice our preliminary data confirm that MR activation in macrophages plays a critical role in the development of vascular stiffness in WD induced insulin resistance and exposure to Aldo • Our data also suggest that MR KO in myeloid cells protects against WD and Aldo-induced vascular stiffness in female mice. • Our results suggest a role for MR activation in PVAT macrophages in the pathogenesis of WD-induced vascular dysfunction

ACKNOWLEDMENTS This research was supported by NIH (R 01 HL 73101 and R 01 HL 107910) and the Veterans Affairs Merit System (0018) for James R. Sowers Mentor: James R. Sowers Collaborators: Luis Martinez-Lemus, Vicent De. Marco, Annayya R Aroor, Guido Lastra, Guanghong Jia Richard Mortensen Laboratory: Dominic Haertling Brady Barron Dongqing Chen Francisco Ramirez University of Missouri University of Missouri University of Michigan
 Raas system
Raas system Muscle tissue where is it found
Muscle tissue where is it found Brown adipose tissue
Brown adipose tissue Define of connective tissue
Define of connective tissue Adipose tissue function
Adipose tissue function Areolar vs reticular connective tissue
Areolar vs reticular connective tissue Collagen fiber
Collagen fiber Cardiac muscle tissue diagram
Cardiac muscle tissue diagram Fat connective tissue function
Fat connective tissue function Adipose tissue in the subcutaneous layer
Adipose tissue in the subcutaneous layer Activation tree example
Activation tree example Star-shaped macrophages that patrol deep epidermis
Star-shaped macrophages that patrol deep epidermis Alveolar macrophages
Alveolar macrophages Macrophages
Macrophages Jaringan epitel dapat ditemukan di
Jaringan epitel dapat ditemukan di Reverse reaction graph
Reverse reaction graph Brand activation meaning
Brand activation meaning Activation energy unit
Activation energy unit Vitronectin
Vitronectin Spreading activation psychology
Spreading activation psychology Brain buttons brain gym
Brain buttons brain gym Activation synthesis theory
Activation synthesis theory Oob grace
Oob grace Rockwell rehost activation
Rockwell rehost activation Scheduler activations in os
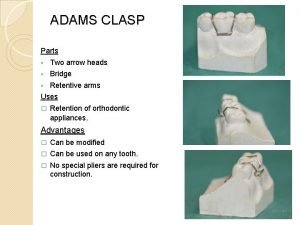
Scheduler activations in os Adams clasp modifications ppt
Adams clasp modifications ppt Allosteric activation
Allosteric activation Diagbox activation code
Diagbox activation code