Mineral dissolutionprecipitation To determine whether or not a

- Slides: 7

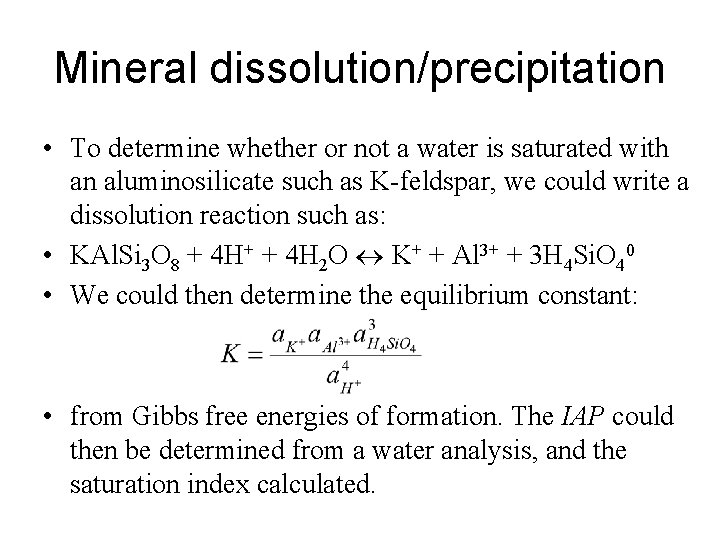
Mineral dissolution/precipitation • To determine whether or not a water is saturated with an aluminosilicate such as K-feldspar, we could write a dissolution reaction such as: • KAl. Si 3 O 8 + 4 H+ + 4 H 2 O K+ + Al 3+ + 3 H 4 Si. O 40 • We could then determine the equilibrium constant: • from Gibbs free energies of formation. The IAP could then be determined from a water analysis, and the saturation index calculated.

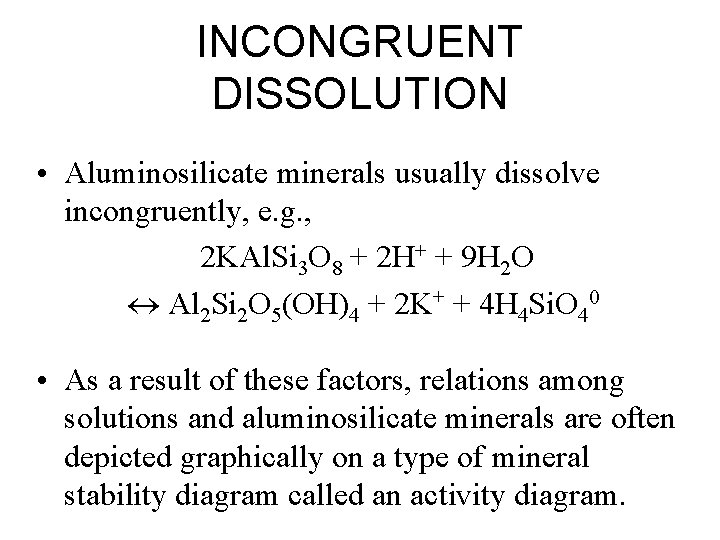
INCONGRUENT DISSOLUTION • Aluminosilicate minerals usually dissolve incongruently, e. g. , 2 KAl. Si 3 O 8 + 2 H+ + 9 H 2 O Al 2 Si 2 O 5(OH)4 + 2 K+ + 4 H 4 Si. O 40 • As a result of these factors, relations among solutions and aluminosilicate minerals are often depicted graphically on a type of mineral stability diagram called an activity diagram.

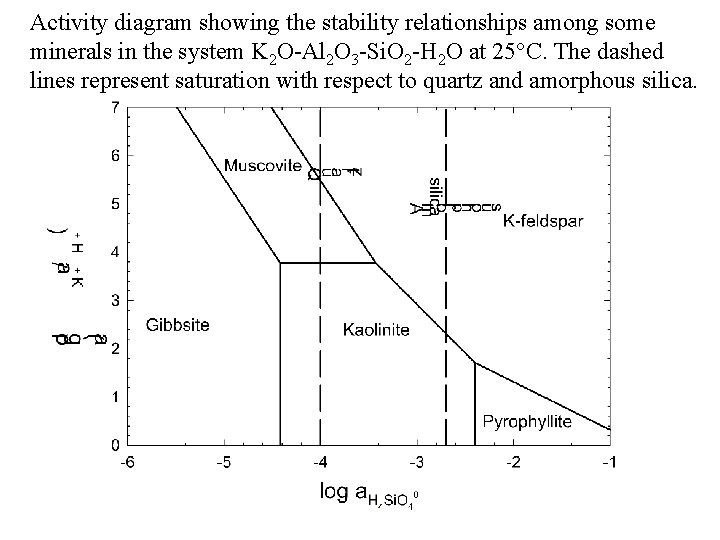
ACTIVITY DIAGRAMS: THE K 2 O-Al 2 O 3 -Si. O 2 -H 2 O SYSTEM We will now calculate an activity diagram for the following phases: gibbsite {Al(OH)3}, kaolinite {Al 2 Si 2 O 5(OH)4}, pyrophyllite {Al 2 Si 4 O 10(OH)2}, muscovite {KAl 3 Si 3 O 10(OH)2}, and K-feldspar {KAl. Si 3 O 8}. The axes will be a K+/a H+ vs. a H 4 Si. O 40. The diagram is divided up into fields where only one of the above phases is stable, separated by straight line boundaries.

Activity diagram showing the stability relationships among some minerals in the system K 2 O-Al 2 O 3 -Si. O 2 -H 2 O at 25°C. The dashed lines represent saturation with respect to quartz and amorphous silica.

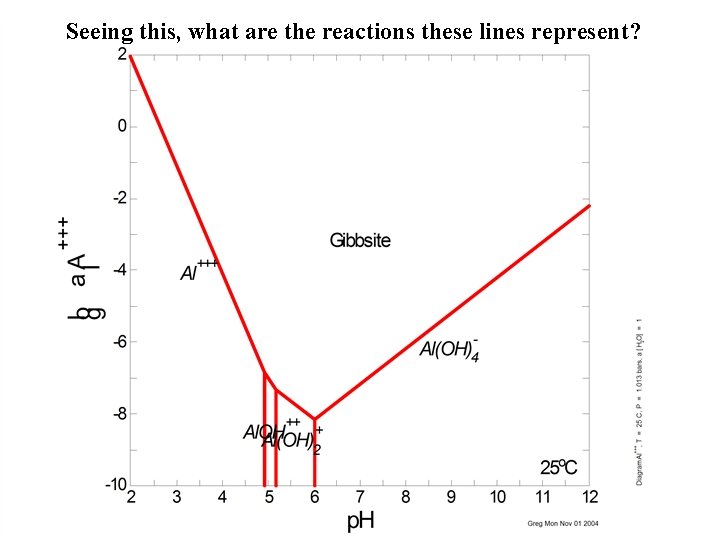
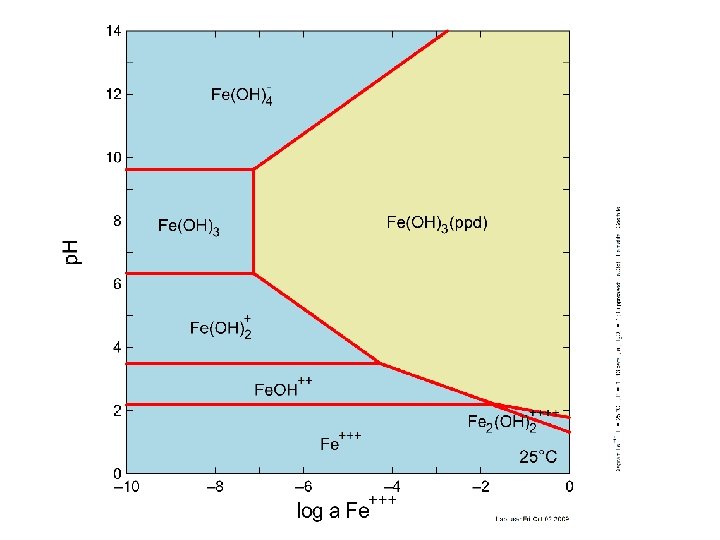
Seeing this, what are the reactions these lines represent?


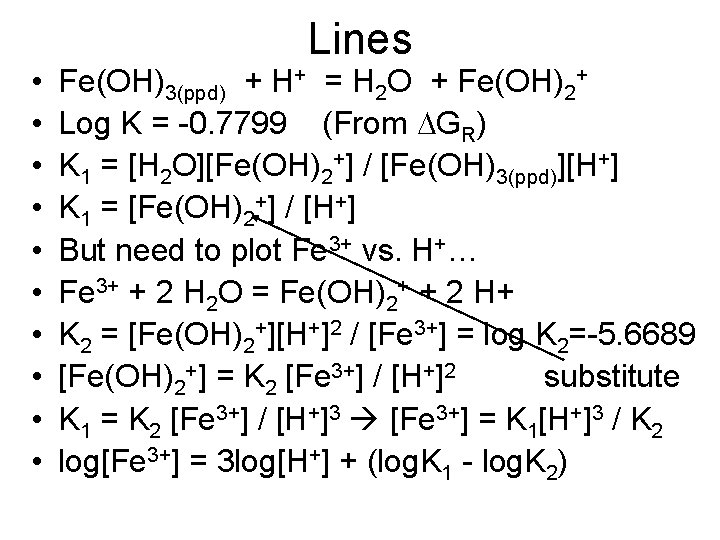
• • • Lines Fe(OH)3(ppd) + H+ = H 2 O + Fe(OH)2+ Log K = -0. 7799 (From DGR) K 1 = [H 2 O][Fe(OH)2+] / [Fe(OH)3(ppd)][H+] K 1 = [Fe(OH)2+] / [H+] But need to plot Fe 3+ vs. H+… Fe 3+ + 2 H 2 O = Fe(OH)2+ + 2 H+ K 2 = [Fe(OH)2+][H+]2 / [Fe 3+] = log K 2=-5. 6689 [Fe(OH)2+] = K 2 [Fe 3+] / [H+]2 substitute K 1 = K 2 [Fe 3+] / [H+]3 [Fe 3+] = K 1[H+]3 / K 2 log[Fe 3+] = 3 log[H+] + (log. K 1 - log. K 2)