Mineral Analysis Gold Analysis Fire Assay Lead Collection





- Slides: 14

Mineral Analysis Gold Analysis – Fire Assay

Lead Collection Fire Assay • A sample of 50 g is weighed into a paper cup. • It is then mixed with a flux that is made up of: – Borax – Soda ash – Lead(II) oxide (litharge) – Silver Nitrate – Silica

• Once the standard flux has been added to the sample, additions of other reagents are used to neutralise the ore: – Extra Silica is added if the ore is basic – Extra Borax is added if the ore is acidic – KNO 3 if the ore is reducing – Flour if the ore is oxidising • At the end of the fusion process, the resulting button should weigh 35 -40 g.

Sulfide ores • If a sample is high in sulfide, a certain amount of KNO 3 and litharge must be added to the flux. – The higher the sulfide content, the more litharge is added. – KNO 3 is added to compensate for the added litharge. • 1 g of KNO 3 will oxidise 4 g of lead. • eg. If 20 g of litharge is added, 5 g of KNO 3 must be added.


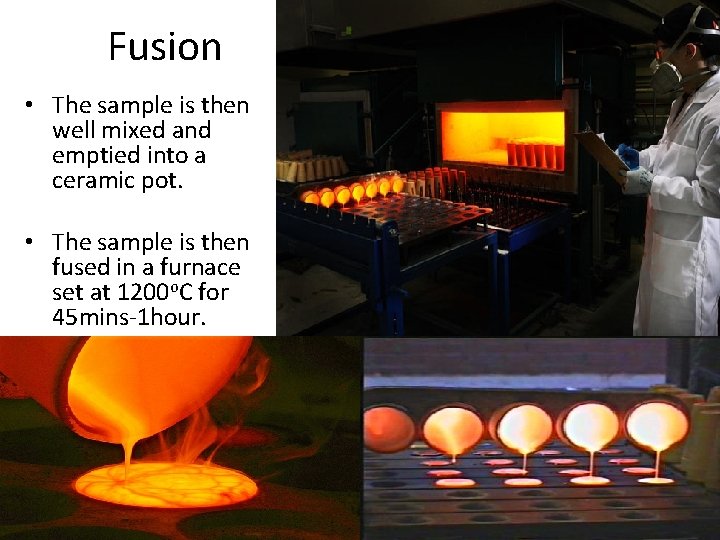
Fusion • The sample is then well mixed and emptied into a ceramic pot. • The sample is then fused in a furnace set at 1200 o. C for 45 mins-1 hour.

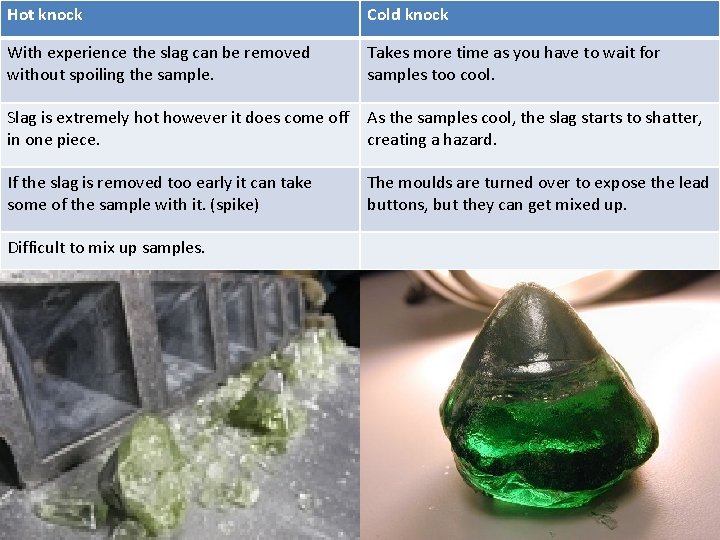
Hot knock Cold knock With experience the slag can be removed without spoiling the sample. Takes more time as you have to wait for samples too cool. Slag is extremely hot however it does come off in one piece. As the samples cool, the slag starts to shatter, creating a hazard. If the slag is removed too early it can take some of the sample with it. (spike) The moulds are turned over to expose the lead buttons, but they can get mixed up. Difficult to mix up samples.

Muffling • The lead buttons are loaded into a muffle furnace at close to 1000 o. C. • The buttons are loaded into clay dishes called “cupels”. • To speed up the process, the cupels can be preheated and the lead buttons loaded with a pair of steel tongs.

• Inside the muffle the lead is oxidised back to litharge which is absorbed into the cupel. • Once muffling is complete, a small gold and silver prill remains in the cupel. No silver, no prill

Analysing the prill • The finish involves either “weighing” or “spraying”. • Weighing involves the use of microbalance to determine the gold in micrograms. • Spraying involves digesting the black gold particles (no annealing is necessary) in 2 m. L of aqua regia, making the digest to volume (10 m. L) and measuring the gold spectrometrically in ppm. – Multiplying the concentration in ppm (mg/L) by the volume gives the gold in micrograms.

Nickel Sulfide Collection • Instead of using a lead based flux, a flux is used that contains nickel oxide and sulphur. • In the fusion furnace these two compounds form nickel sulfide which collects platinum gold elements (PGE’s). • Once the sample is poured, the resulting nickel sulfide button (which is brittle) is pulverised into powder.

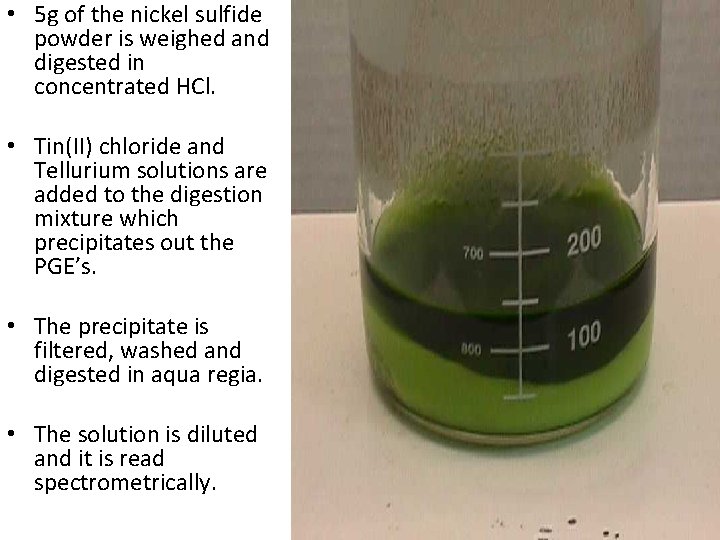
• 5 g of the nickel sulfide powder is weighed and digested in concentrated HCl. • Tin(II) chloride and Tellurium solutions are added to the digestion mixture which precipitates out the PGE’s. • The precipitate is filtered, washed and digested in aqua regia. • The solution is diluted and it is read spectrometrically.

Interferences Ø Sulfide: forms a hard brittle substance around the top of the button called “matte” which shatters incurring loss of sample. Ø Nickel: Whilst muffling, high nickel samples form a shell on top of the sample called a “scoria”. Ø Copper: also forms a scoria at high concentrations.

• Next topic “Gold Analysis – Aqua Regia”