Mineral Analysis Gold Analysis Aqua Regia Gold is









- Slides: 14

Mineral Analysis Gold Analysis – Aqua Regia

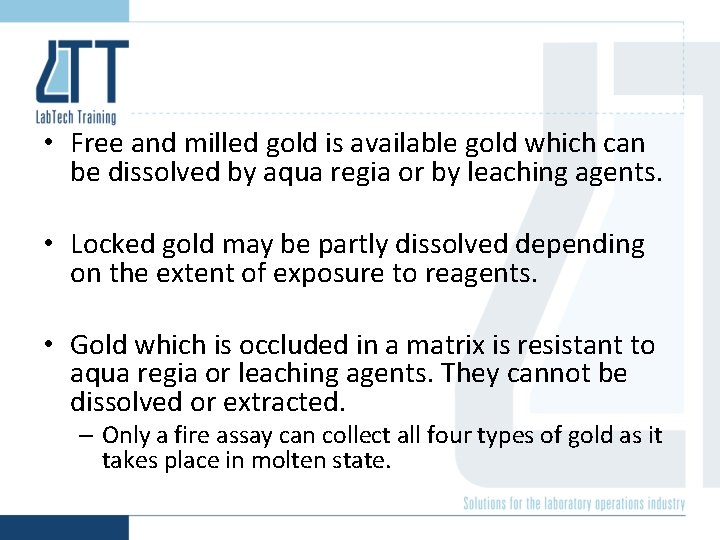
Gold is physically present in ores in one of four ways: 1) as free gold 2) as milled gold 3) as locked gold 4) as occluded gold

• Free and milled gold is available gold which can be dissolved by aqua regia or by leaching agents. • Locked gold may be partly dissolved depending on the extent of exposure to reagents. • Gold which is occluded in a matrix is resistant to aqua regia or leaching agents. They cannot be dissolved or extracted. – Only a fire assay can collect all four types of gold as it takes place in molten state.

Aqua Regia Digestion This involves a 15 to 40 g ore sample which is digested for 1 ½ - 2 hours in Aqua Regia (which is a mixture of concentrated HCl and HNO 3) and made up to volume.

If the ore is high in sulphur, a pre-digestion is required so that the nitric acid in the aqua regia is not consumed before it reacts with the gold. S(s) + 6 HNO 3(aq) 2 H 2 O(l) + H 2 SO 4(aq) + 6 NO 2(g)

Addition of Nitric Acid: Au (s) + 3 NO 3 - (aq) + 6 H+ (aq) → Au 3+ (aq) + 3 NO 2 (g) + 3 H 2 O (l) Addition of Hydrochloric Acid: Au 3+ (aq) + 4 Cl- (aq) + H+ (aq) → HAu. Cl 4 (aq)

Solvent Extraction • If Au<20 ppm, solvent extraction will be necessary prior to AAS analysis. • HAu. Cl 4 is more soluble in ketones than in water. • The extraction criteria must be the same for samples and standards. • In commercial laboratories, samples and standards are shaken in a box containing up to 60 test tubes.

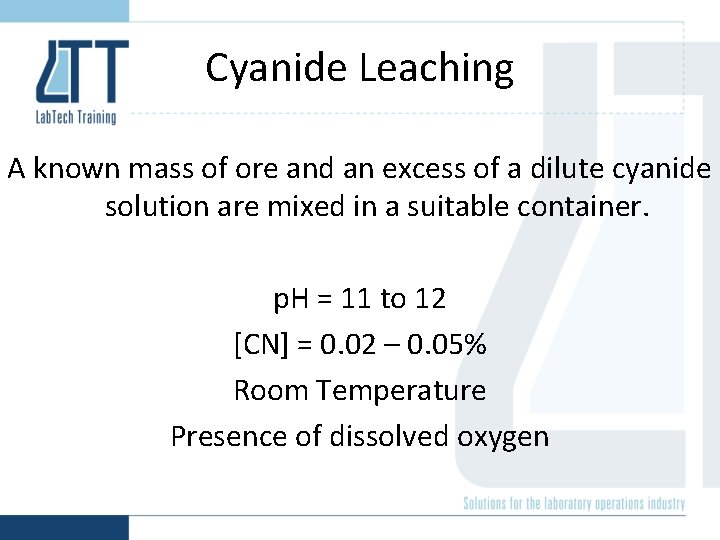
Cyanide Leaching A known mass of ore and an excess of a dilute cyanide solution are mixed in a suitable container. p. H = 11 to 12 [CN] = 0. 02 – 0. 05% Room Temperature Presence of dissolved oxygen

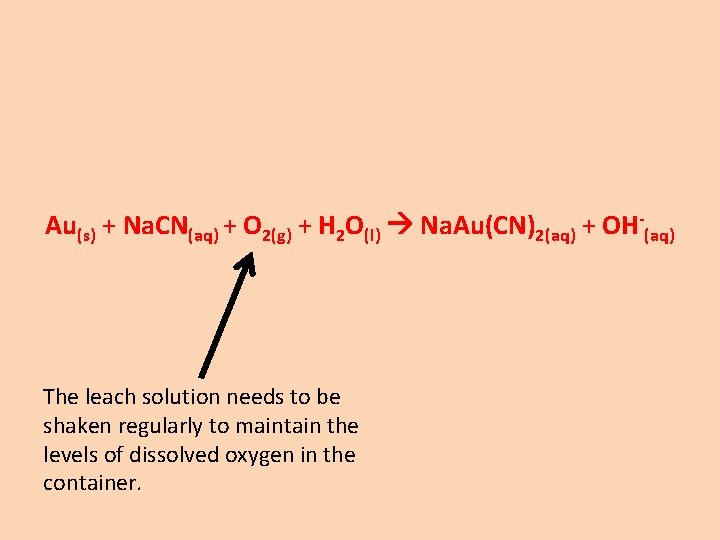
Au(s) + Na. CN(aq) + O 2(g) + H 2 O(l) Na. Au(CN)2(aq) + OH-(aq) The leach solution needs to be shaken regularly to maintain the levels of dissolved oxygen in the container.

Cyanide is extremely toxic. Ingesting 200 mg of potassium cyanide is fatal! • The following safety practices need to be observed: – Always ensure that the p. H is around 11. Below a p. H of 9. 5 KCN reverts to gaseous toxic hydrogen cyanide, HCN – Wear a gas mask and safety glasses when opening the bottles – Wash your hands after working with cyanide – Keep acid bottles away from cyanide!!!

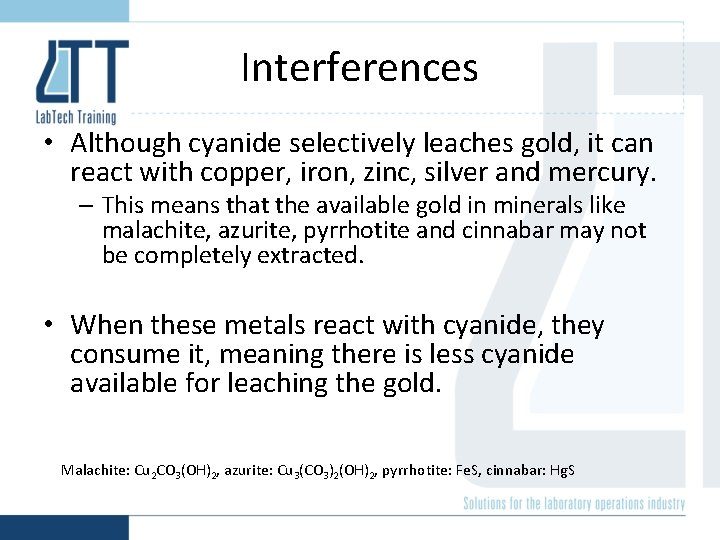
Interferences • Although cyanide selectively leaches gold, it can react with copper, iron, zinc, silver and mercury. – This means that the available gold in minerals like malachite, azurite, pyrrhotite and cinnabar may not be completely extracted. • When these metals react with cyanide, they consume it, meaning there is less cyanide available for leaching the gold. Malachite: Cu 2 CO 3(OH)2, azurite: Cu 3(CO 3)2(OH)2, pyrrhotite: Fe. S, cinnabar: Hg. S

• When Fe(II) is converted to Fe(III), the reaction consumes oxygen. To compensate the leach solution must be shaken more often. • In the presence of a highly sulphide ore, the cyanide may be consumed to form thiocyanate (SCN-). • The presence of small amounts of Pb, Hg, Bi, and Ti accelerate dissolution. Adding an amount of lead nitrate can improve the speed of the leaching process.

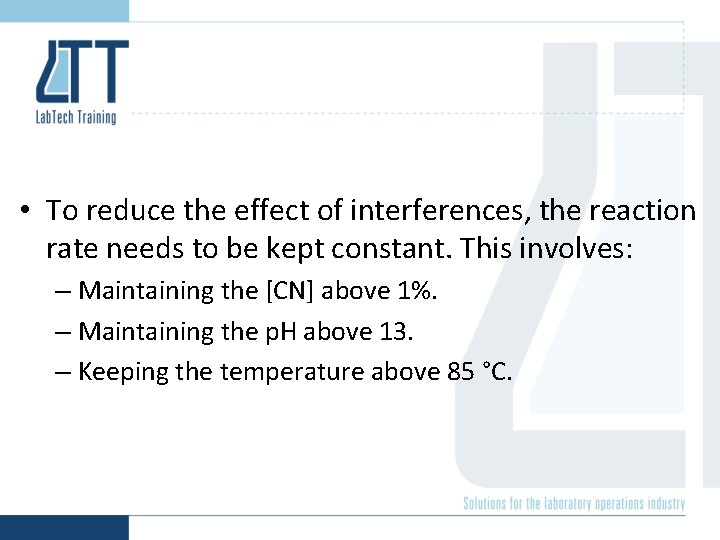
• To reduce the effect of interferences, the reaction rate needs to be kept constant. This involves: – Maintaining the [CN] above 1%. – Maintaining the p. H above 13. – Keeping the temperature above 85 °C.

1. Adding sample 2. Adding cyanide and ball bearings 4. Sealing pots 3. Adding water