Mineral Analysis Data Evaluation Data Evaluation Involves calculation


- Slides: 12

Mineral Analysis Data Evaluation

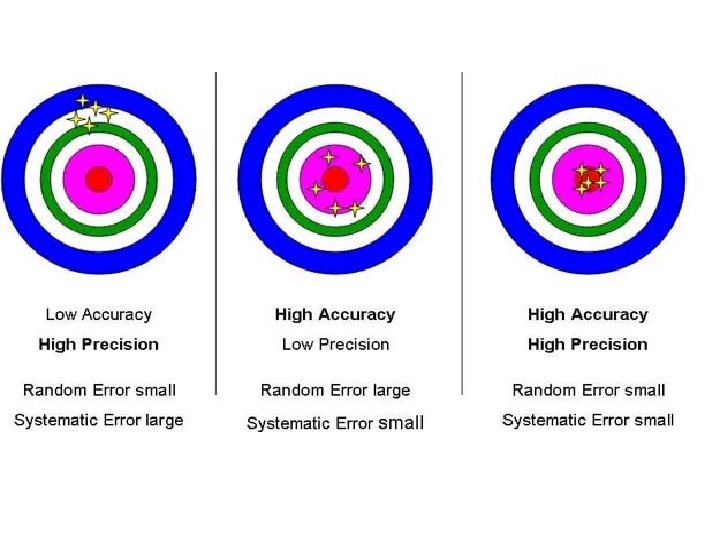
Data Evaluation • Involves calculation of the principal result and the precision. The principal result should be accurate. • Accuracy is how close a measured value is to the actual (true) value. • Precision is how close the measured values are to each other.


• Poor precision arises from: – Uncertainties due to sampling – The chemical behaviour of the sample during digestion and measurement – The behaviour of the instruments – Operator errors • Precision checks or “duplicates” are normally added to sample batches to monitor the precision of the results.

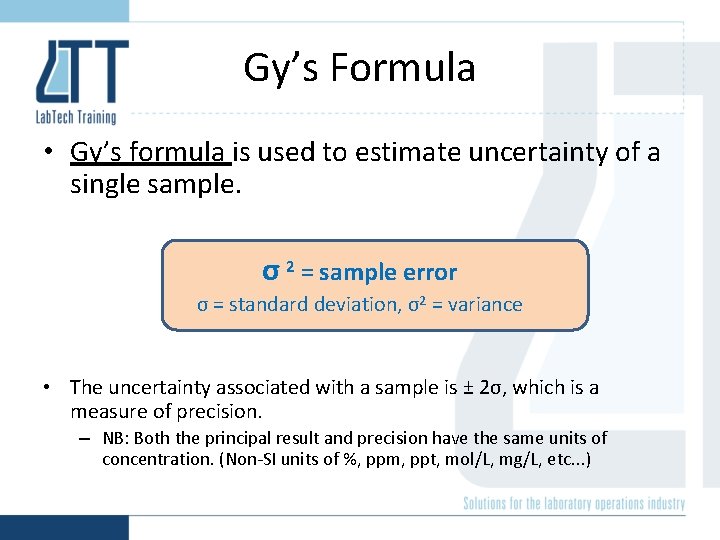
Gy’s Formula • Gy’s formula is used to estimate uncertainty of a single sample. σ 2 = sample error σ = standard deviation, σ2 = variance • The uncertainty associated with a sample is ± 2σ, which is a measure of precision. – NB: Both the principal result and precision have the same units of concentration. (Non-SI units of %, ppm, ppt, mol/L, mg/L, etc. . . )

• Calculation of the result takes into account: – the sample mass, – dilution factors, and – pre-concentration factors (if any).

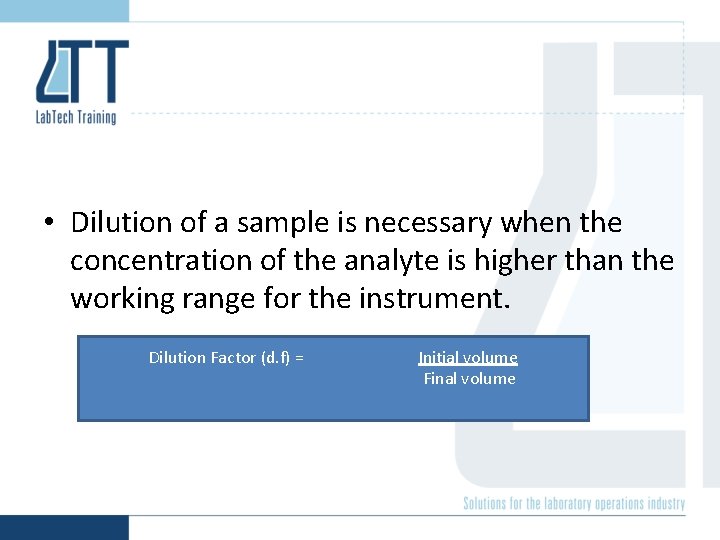
• Dilution of a sample is necessary when the concentration of the analyte is higher than the working range for the instrument. Dilution Factor (d. f) = Initial volume Final volume

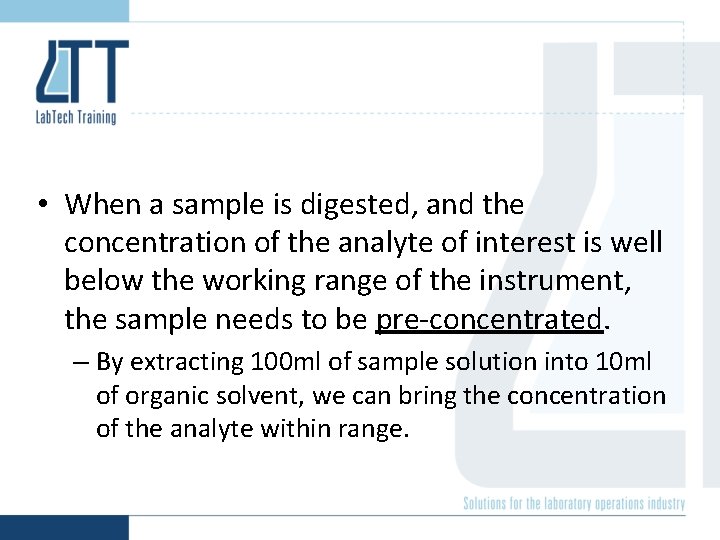
• When a sample is digested, and the concentration of the analyte of interest is well below the working range of the instrument, the sample needs to be pre-concentrated. – By extracting 100 ml of sample solution into 10 ml of organic solvent, we can bring the concentration of the analyte within range.

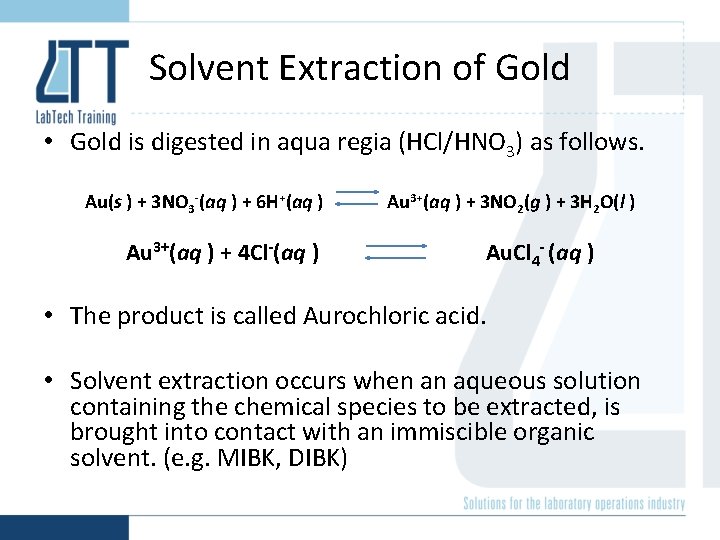
Solvent Extraction of Gold • Gold is digested in aqua regia (HCl/HNO 3) as follows. Au(s ) + 3 NO 3 -(aq ) + 6 H+(aq ) Au 3+(aq ) + 4 Cl-(aq ) Au 3+(aq ) + 3 NO 2(g ) + 3 H 2 O(l ) Au. Cl 4 - (aq ) • The product is called Aurochloric acid. • Solvent extraction occurs when an aqueous solution containing the chemical species to be extracted, is brought into contact with an immiscible organic solvent. (e. g. MIBK, DIBK)

• In solvent extraction like dissolves like so HAu. Cl 4 is soluble in ketone (MIBK). • All the Au is transferred to the 10 m. L organic layer from the 100 m. L aqueous layer. (10 x preconcentration factor) This is the basis of solvent extraction as a preconcentration technique.

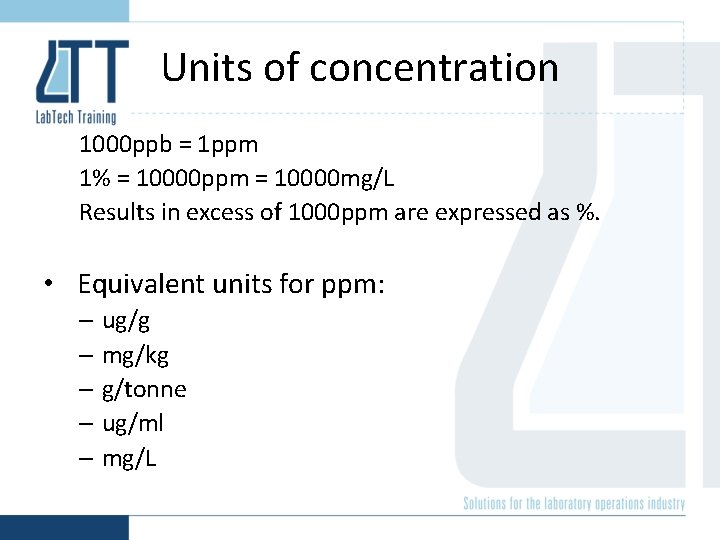
Units of concentration 1000 ppb = 1 ppm 1% = 10000 ppm = 10000 mg/L Results in excess of 1000 ppm are expressed as %. • Equivalent units for ppm: – ug/g – mg/kg – g/tonne – ug/ml – mg/L

• Next topic “The Dilution Formula”