Mielodisplasie Utilizzo dei chelanti del ferro M D




















- Slides: 49

Mielodisplasie Utilizzo dei chelanti del ferro M. D. Cappellini Fondazione Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena IRCCS Università di Milano Corso Nazionale di Aggiornamento in Ematologia Clinica Roma , Ambasciatori Palace Hotel 8 -9 Novembre 2006

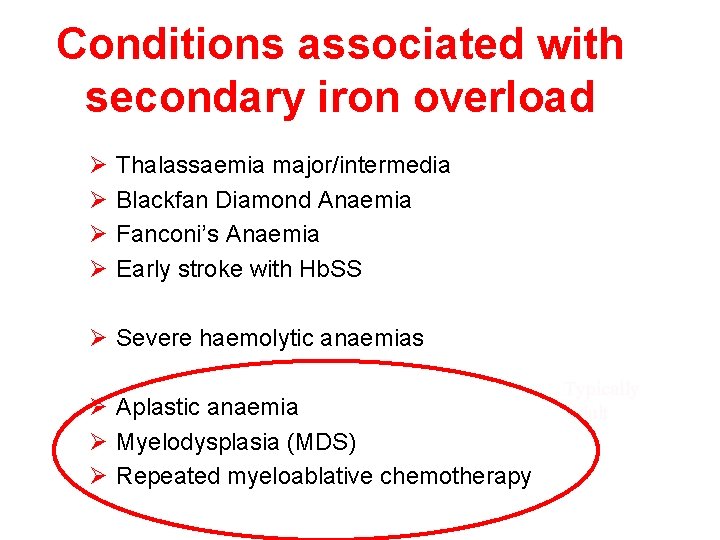
Conditions associated with secondary iron overload Ø Ø Thalassaemia major/intermedia Blackfan Diamond Anaemia Fanconi’s Anaemia Early stroke with Hb. SS Ø Severe haemolytic anaemias Ø Aplastic anaemia Ø Myelodysplasia (MDS) Ø Repeated myeloablative chemotherapy Typically adult

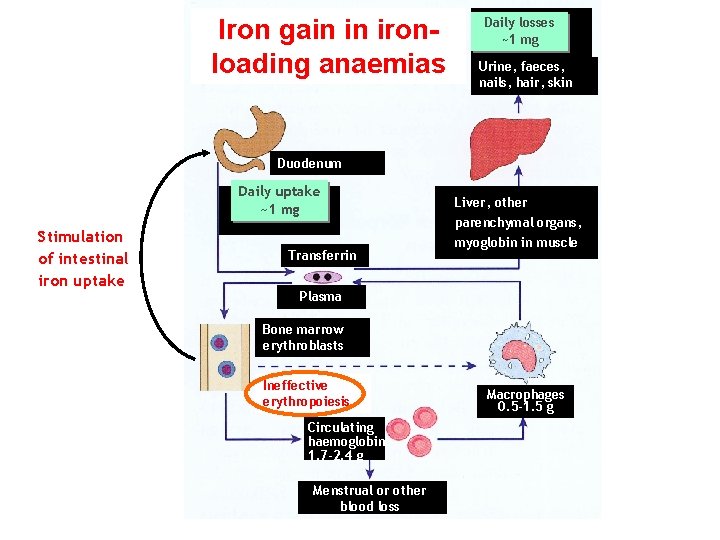
Iron gain in ironloading anaemias Daily losses ~1 mg Urine, faeces, nails, hair, skin Duodenum Daily uptake ~1 mg Stimulation of intestinal iron uptake Transferrin Liver, other parenchymal organs, myoglobin in muscle Plasma Bone marrow erythroblasts Ineffective erythropoiesis Circulating haemoglobin 1. 7 -2. 4 g Menstrual or other blood loss Macrophages 0. 5 -1. 5 g

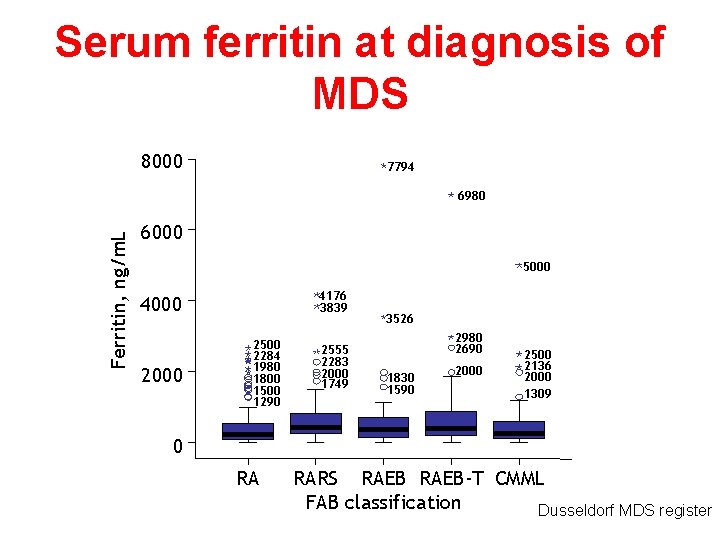
Serum ferritin at diagnosis of MDS 8000 7794 Ferritin, ng/m. L 6980 6000 5000 4176 3839 4000 2500 2284 1980 1800 1500 1290 2555 2283 2000 1749 3526 2980 2690 1830 1590 2000 2500 2136 2000 1309 0 RA RARS RAEB-T CMML FAB classification Dusseldorf MDS register

Iron balance in transfusion Daily losses 1 mg 1 blood unit 200– 250 mg

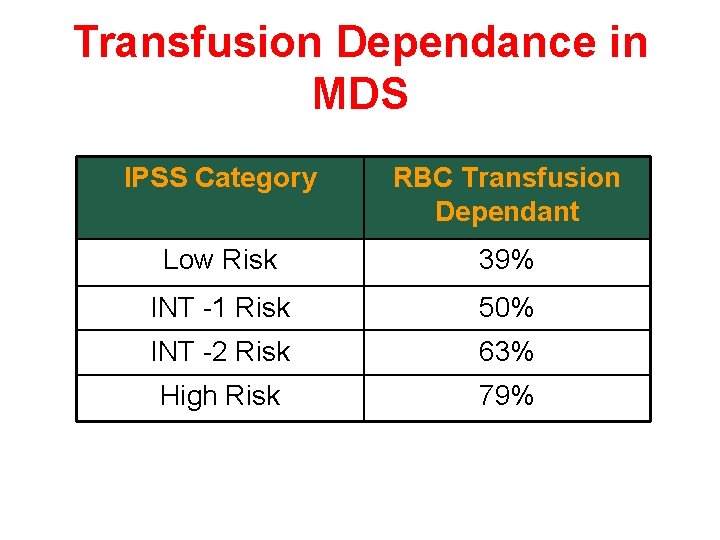
Transfusion Dependance in MDS IPSS Category RBC Transfusion Dependant Low Risk 39% INT -1 Risk 50% INT -2 Risk 63% High Risk 79%

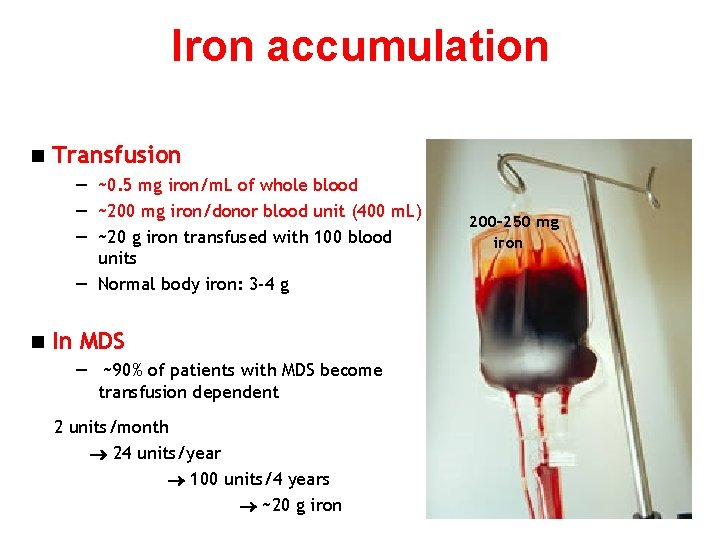
Iron accumulation n Transfusion — ~0. 5 mg iron/m. L of whole blood — ~200 mg iron/donor blood unit (400 m. L) — ~20 g iron transfused with 100 blood units — Normal body iron: 3 -4 g n In MDS — ~90% of patients with MDS become transfusion dependent 2 units/month 24 units/year 100 units/4 years ~20 g iron 200– 250 mg iron

Iron turnover in transfusional overload Erythron Transfusion Parenchyma 20– 40 mg/day (0. 3– 0. 7 mg/kg/day) Parenchyma Hepatocytes NTBI Red Transferrin Macrophages Gut Porter JB. Hematol Oncol Clin North Am. 2005; 19: 1 -6.

Consequences of Iron-Mediated Toxicity Increased “free” iron Hydroxyl radical generation Organelle damage Lipid peroxidation Lysosomal fragility TGF-β 1 Collagen synthesis Enzyme leakage Cell death Fibrosis TGF = transforming growth factor Adapted from Porter JB. Hematol Oncol Clin North Am. 2005; 19(suppl 1): 7 -12, with permission from Elsevier.

Organs susceptible to iron overload Clinical sequelae of iron overload Pituitary → impaired growth Heart → cardiomyopathy, cardiac failure Liver → hepatic cirrhosis Pancreas → diabetes mellitus Gonads → hypogonadism, infertility

Organs susceptible to iron overload Clinical sequelae of iron overload Pituitary → impaired growth Heart → cardiomyopathy, cardiac failure Liver → hepatic cirrhosis Pancreas → diabetes mellitus Gonads → hypogonadism, infertility

Patients with cardiac iron deposits at post-mortem in the pre-chelation era Patients with cardiac iron (%) • 131 transfused adult patients – – 100 101 leukaemias 30 other anaemias 80 60 40 20 0 0– 25 26– 50 51– 75 76– 100 101– 200 201– 300 Units of blood transfused Buja LM, Roberts WC. Am J Med. 1971; 51: 209 -21.

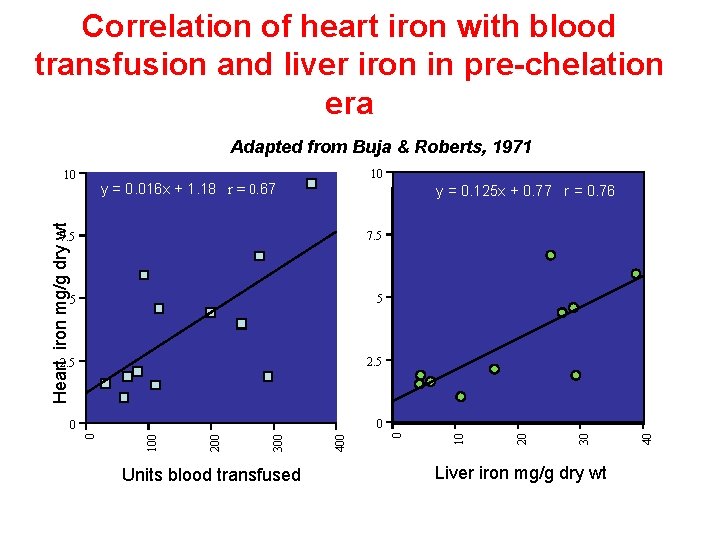
Correlation of heart iron with blood transfusion and liver iron in pre-chelation era Adapted from Buja & Roberts, 1971 10 y = 0. 016 x + 1. 18 r = 0. 67 Units blood transfused Liver iron mg/g dry wt 40 0 30 0 20 2. 5 400 2. 5 300 5 200 5 100 7. 5 10 y = 0. 125 x + 0. 77 r = 0. 76 0 Heart iron mg/g dry wt 10

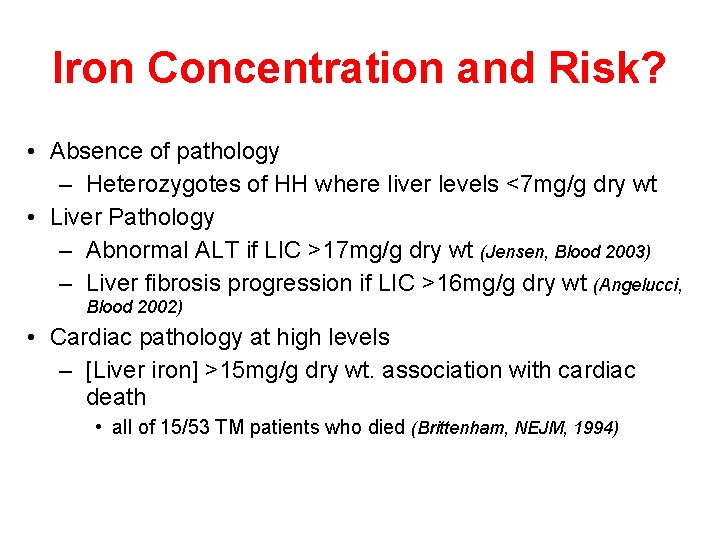
Iron Concentration and Risk? • Absence of pathology – Heterozygotes of HH where liver levels <7 mg/g dry wt • Liver Pathology – Abnormal ALT if LIC >17 mg/g dry wt (Jensen, Blood 2003) – Liver fibrosis progression if LIC >16 mg/g dry wt (Angelucci, Blood 2002) • Cardiac pathology at high levels – [Liver iron] >15 mg/g dry wt. association with cardiac death • all of 15/53 TM patients who died (Brittenham, NEJM, 1994)

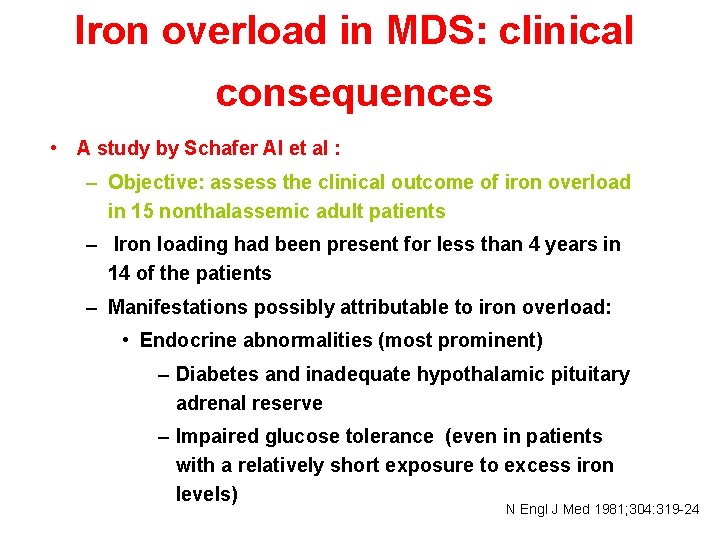
Iron overload in MDS: clinical consequences • A study by Schafer AI et al : – Objective: assess the clinical outcome of iron overload in 15 nonthalassemic adult patients – Iron loading had been present for less than 4 years in 14 of the patients – Manifestations possibly attributable to iron overload: • Endocrine abnormalities (most prominent) – Diabetes and inadequate hypothalamic pituitary adrenal reserve – Impaired glucose tolerance (even in patients with a relatively short exposure to excess iron levels) N Engl J Med 1981; 304: 319 -24

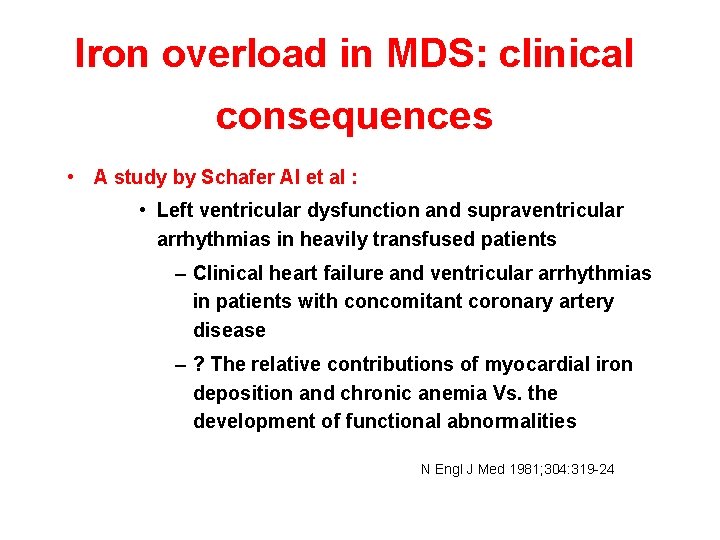
Iron overload in MDS: clinical consequences • A study by Schafer AI et al : • Left ventricular dysfunction and supraventricular arrhythmias in heavily transfused patients – Clinical heart failure and ventricular arrhythmias in patients with concomitant coronary artery disease – ? The relative contributions of myocardial iron deposition and chronic anemia Vs. the development of functional abnormalities N Engl J Med 1981; 304: 319 -24

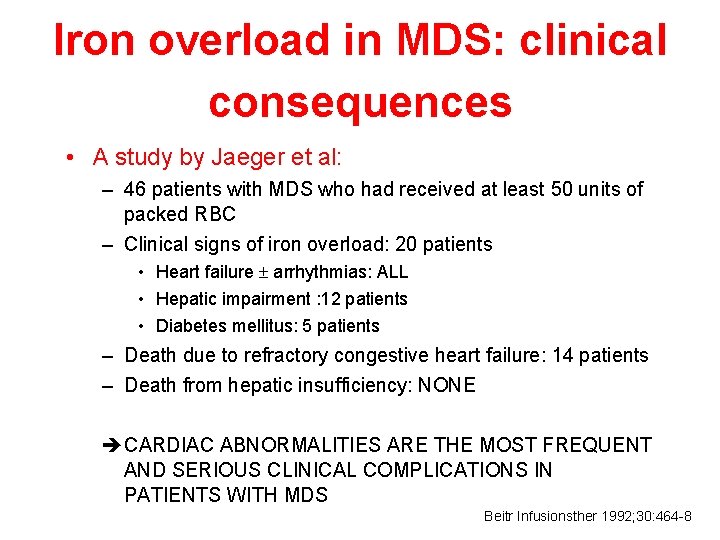
Iron overload in MDS: clinical consequences • A study by Jaeger et al: – 46 patients with MDS who had received at least 50 units of packed RBC – Clinical signs of iron overload: 20 patients • Heart failure arrhythmias: ALL • Hepatic impairment : 12 patients • Diabetes mellitus: 5 patients – Death due to refractory congestive heart failure: 14 patients – Death from hepatic insufficiency: NONE è CARDIAC ABNORMALITIES ARE THE MOST FREQUENT AND SERIOUS CLINICAL COMPLICATIONS IN PATIENTS WITH MDS Beitr Infusionsther 1992; 30: 464 -8

Other Reports of Iron Overload in MDS Author Year # of Pts Organ Damage Hepatic fibrosis, LIC LV dysfunction, arrhythmia Glucose intolerance Pituitary-adrenal reserve Schafer et al 1981 10 8 15 10 Walterova 1983 8 Cazzola 1988 14 18 Abn GTT (6 DM) Abn LFT’s (1 died of cirrhosis) Jaeger 1992 20 11 5 Frame 1994 1 deceased 20 cardiac failure arrhythmias, CHF hepatic fibrosis, abn LFT’s diabetes mellitus RARS subtype – most complications Gynecomastia, skin bronzing, increasing transfusion requirement

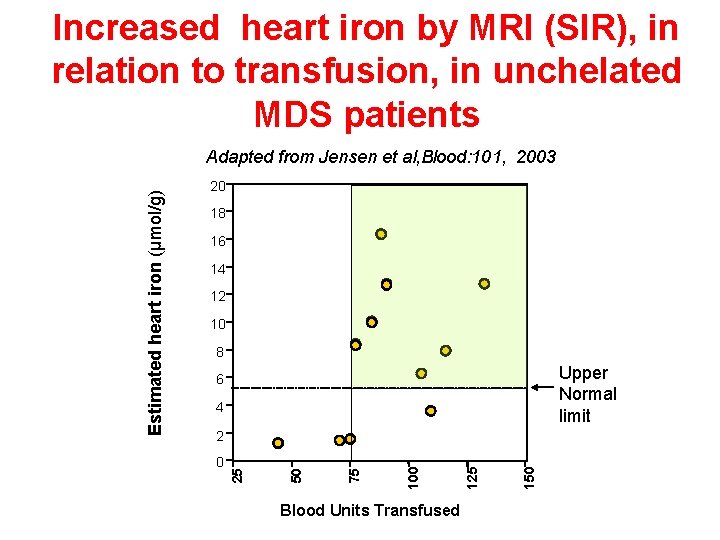
Increased heart iron by MRI (SIR), in relation to transfusion, in unchelated MDS patients 20 18 16 14 12 10 8 Upper Normal limit 6 4 Blood Units Transfused 150 125 100 75 0 50 2 25 Estimated heart iron (µmol/g) Adapted from Jensen et al, Blood: 101, 2003

Iron chelation in MDS • Do we need iron chelation therapy for patients with MDS? • Which patients with MDS may benefit from iron chelation? • Which chelator can be used?

Secondary iron overload in transfusiondependent MDS patients L. Malcovati, 2005

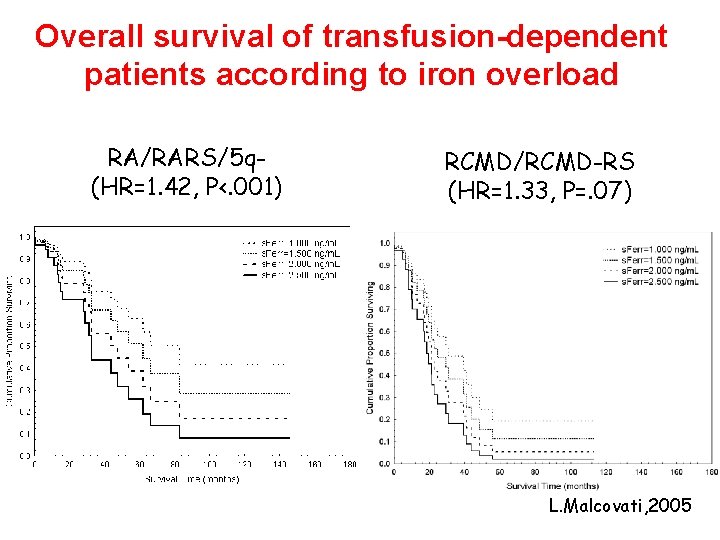
Overall survival of transfusion-dependent patients according to iron overload RA/RARS/5 q(HR=1. 42, P<. 001) RCMD/RCMD-RS (HR=1. 33, P=. 07) L. Malcovati, 2005

Probability of non-leukemic death in MDS patients according to transfusiondependency MDS without excess blasts (HR=1. 7, P=. 03) MDS with excess blasts (P=. 38) L. Malcovati, 2005

Iron Overload in MDS Consensus Meeting Nagasaki, Japan May 11, 2005

Consensus reached Questions • How frequently would you monitor iron overload? Consensus • At least every 3 months in patients receiving transfusion • Which tools would you use to • diagnose and monitor iron overload? • • Serum ferritin Transferrin saturation Liver MRI • What would you use most frequently? • Serum ferritin > 1, 000– 2, 000 ng/m. L considering rate of transfusions • As long as transfusion therapy continues (as long as iron overload is clinically relevant) • When would you start chelation therapy? • For how long would you continue chelation therapy? MRI = magnetic resonance imaging. Hematol Oncol Clin North Am. 2005; 19 Suppl 1.

Consensus reached Questions • What is the profile of a patient who might benefit from the treatment of iron overload? Consensus • Transfusion-dependent patients • Low risk MDS: IPSS low or Int-1 • WHO-type RA and RARS and 5 q– syndrome • Candidates for allografting • MDS patients with documented stable disease • Serum ferritin levels > 1, 000– 2, 000 ng/m. L or other evidence of significant tissue iron overload • Absence of comorbidities severely limiting prognosis

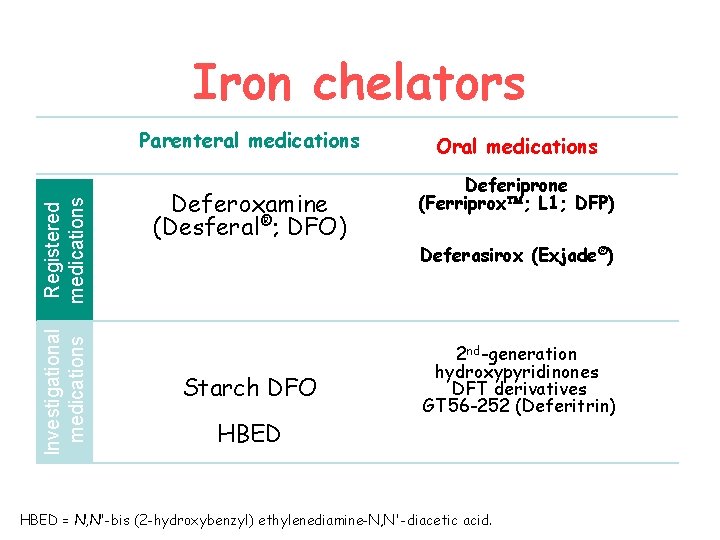
Iron chelators Investigational medications Registered medications Parenteral medications Deferoxamine (Desferal®; DFO) Starch DFO HBED Oral medications Deferiprone (Ferriprox ; L 1; DFP) Deferasirox (Exjade®) 2 nd-generation hydroxypyridinones DFT derivatives GT 56 -252 (Deferitrin) HBED = N, N'-bis (2 -hydroxybenzyl) ethylenediamine-N, N'-diacetic acid.

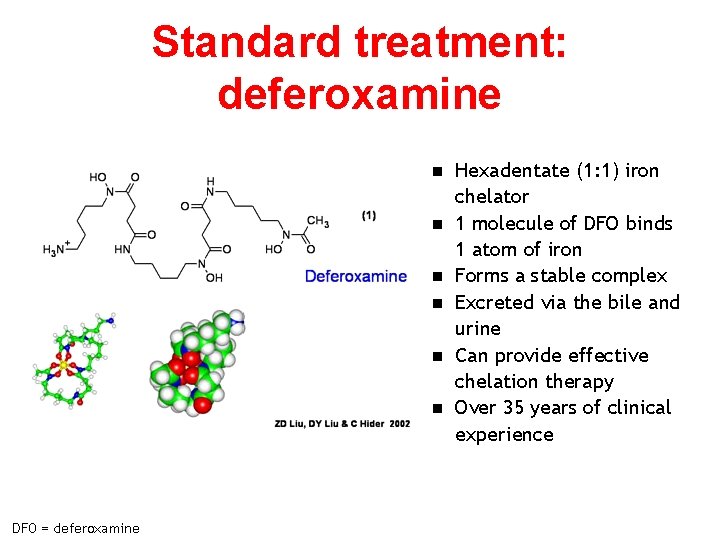
Standard treatment: deferoxamine n n n DFO = deferoxamine Hexadentate (1: 1) iron chelator 1 molecule of DFO binds 1 atom of iron Forms a stable complex Excreted via the bile and urine Can provide effective chelation therapy Over 35 years of clinical experience

The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload P. D. JENSEN, 1 L. HEICKENDORFF, 2 B. PEDERSEN, 3 K. BENDIX-HANSEN, 4 F. T. JENSEN, 5 T. CHRISTENSEN, 5 A. M. BOESEN 1 AND J. ELLEGAARD 1 1 Department of Medicine and Haematology, 2 Department of Clinical Biochemistry, 3 Department of Cytogenetics, Danish Cancer Society , 4 Institute of Pathology, Aarhus Amtssygehus, and 5 Centre for Nuclear Magnetic Resonance, Skejby Sygehus, Aarhus University Hospital, Denmark Br J Haematol 1996; 94: 288 -99

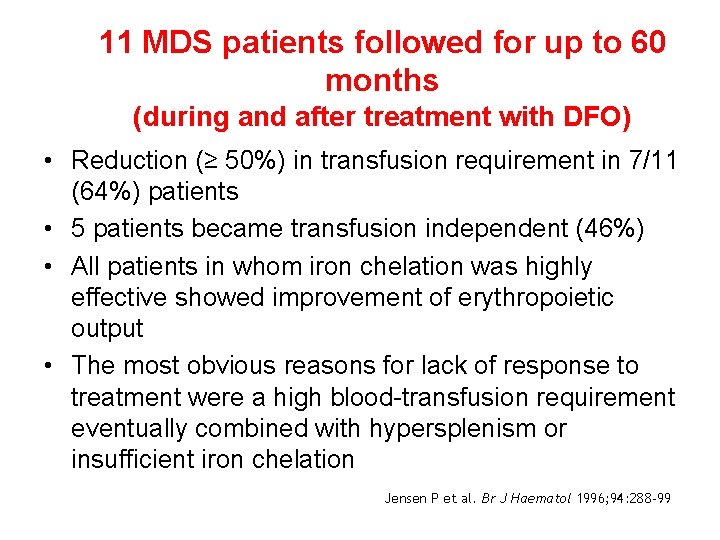
11 MDS patients followed for up to 60 months (during and after treatment with DFO) • Reduction (≥ 50%) in transfusion requirement in 7/11 (64%) patients • 5 patients became transfusion independent (46%) • All patients in whom iron chelation was highly effective showed improvement of erythropoietic output • The most obvious reasons for lack of response to treatment were a high blood-transfusion requirement eventually combined with hypersplenism or insufficient iron chelation Jensen P et al. Br J Haematol 1996; 94: 288 -99

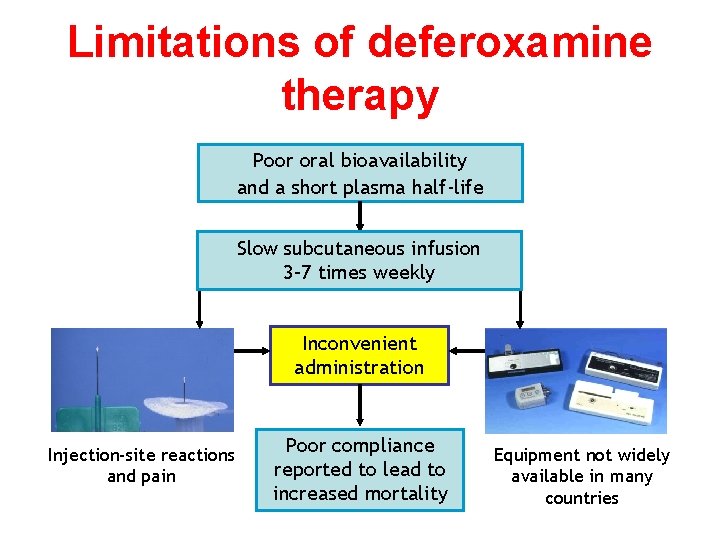
Limitations of deferoxamine therapy Poor oral bioavailability and a short plasma half-life Slow subcutaneous infusion 3– 7 times weekly Inconvenient administration Injection-site reactions and pain Poor compliance reported to lead to increased mortality Equipment not widely available in many countries

DFP (Ferriprox) History • Patented 1982; licensed in EU 1999 Pharmacology Side-effects • Neutropenia/agranulocytosis – weekly neutrophil count recommended 1 • Nausea, vomiting, abdominal pain Efficacy • Arthralgia and arthritis (variable; 4– 50%) • Indicated for “treatment of iron overload • Bidentate, short plasma half-life, given 3 times/day, rapidly glucuronidated • Urinary excretion in patients with thalassemia major when deferoxamine therapy is contra-indicated or inadequate” 1 • May be less effective than DFO in reducing LIC – low efficiency (7%) • Possible cardioprotective effect 8

Iron overload in polytransfused patients with MDS: use of L 1 for oral iron chelation M. JAEGER, C. AUL, D. SÖHNGEN, U. GERMING, AND W. SCHNEIDER Haematology and Oncology Division, Department of Internal Medicine, Heinrich Heine University, Moorenstrasse 5, 4000 Düsseldorf 1, Germany Drugs Today 1992; 28(Suppl A): 143 -7

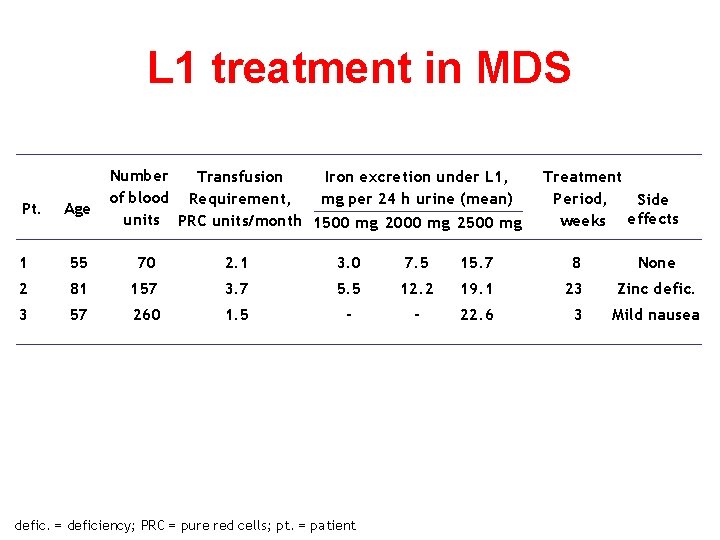
L 1 treatment in MDS Pt. Age Number Transfusion Iron excretion under L 1, of blood Requirement, mg per 24 h urine (mean) units PRC units/month 1500 mg 2000 mg 2500 mg Treatment Period, Side weeks effects 1 55 70 2. 1 3. 0 7. 5 15. 7 8 None 2 81 157 3. 7 5. 5 12. 2 19. 1 23 Zinc defic. 3 57 260 1. 5 - - 22. 6 3 Mild nausea defic. = deficiency; PRC = pure red cells; pt. = patient

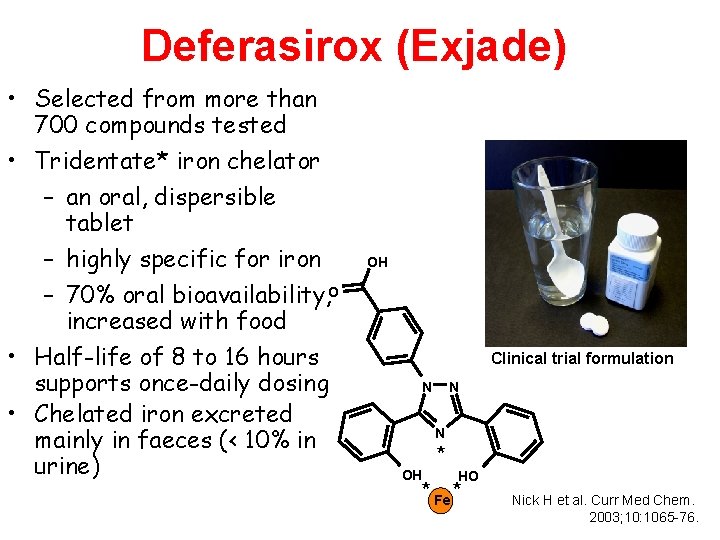
Deferasirox (Exjade) • Selected from more than 700 compounds tested • Tridentate* iron chelator – an oral, dispersible tablet – highly specific for iron – 70% oral bioavailability, O increased with food • Half-life of 8 to 16 hours supports once-daily dosing • Chelated iron excreted mainly in faeces (< 10% in urine) OH Clinical trial formulation N N N OH * HO * Fe * Nick H et al. Curr Med Chem. 2003; 10: 1065 -76.

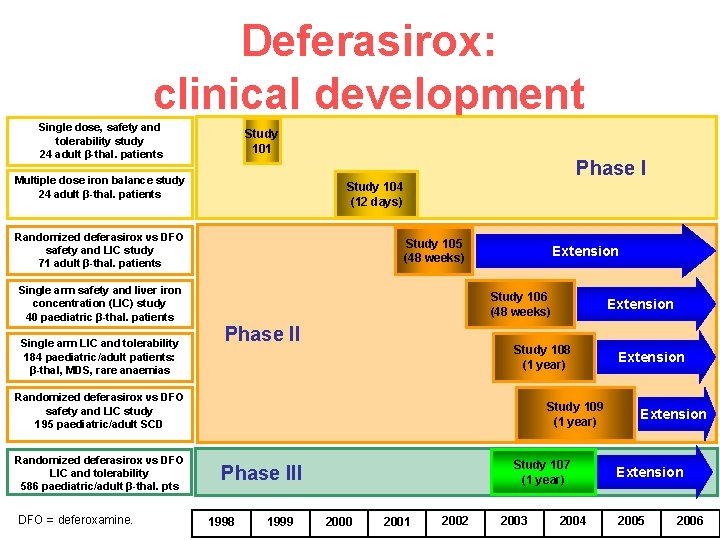
Deferasirox: clinical development Single dose, safety and tolerability study 24 adult β-thal. patients Study 101 Phase I Multiple dose iron balance study 24 adult β-thal. patients Study 104 (12 days) Randomized deferasirox vs DFO safety and LIC study 71 adult β-thal. patients Single arm safety and liver iron concentration (LIC) study 40 paediatric β-thal. patients Single arm LIC and tolerability 184 paediatric/adult patients: β-thal, MDS, rare anaemias Study 105 (48 weeks) Extension Study 106 (48 weeks) Phase II Study 108 (1 year) Randomized deferasirox vs DFO safety and LIC study 195 paediatric/adult SCD Randomized deferasirox vs DFO LIC and tolerability 586 paediatric/adult β-thal. pts DFO = deferoxamine. Extension Study 109 (1 year) Study 107 (1 year) Phase III 1998 1999 2000 2001 2002 2003 2004 Extension 2005 2006

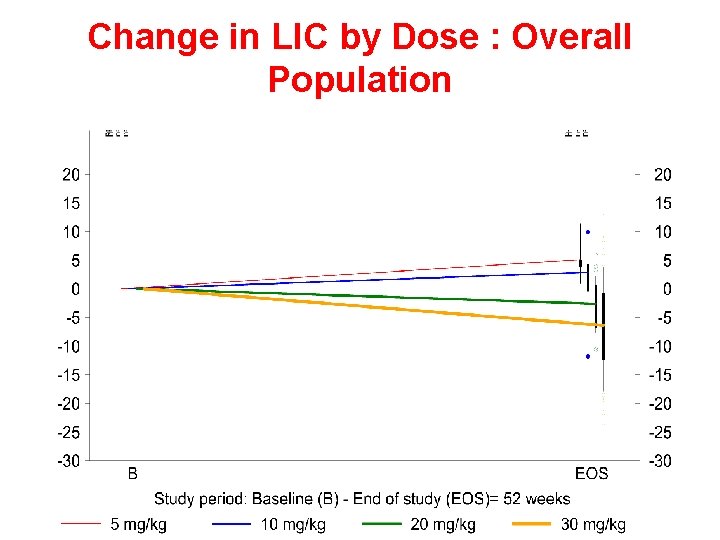
Efficacy results: Study 107 Change in LIC by dose group Change in LIC (mg Fe/g dry weight) 10 5 0 – 5 – 10 DFO, mg/kg/day – 15 Deferasirox, mg/kg/day – 20 DFO Deferasirox n < 25 5 13 15 25–<35 10 75 68 35– 50 20 87 77 ≥ 50 30 98 108 Cappellini MD, et al Blood. 2006

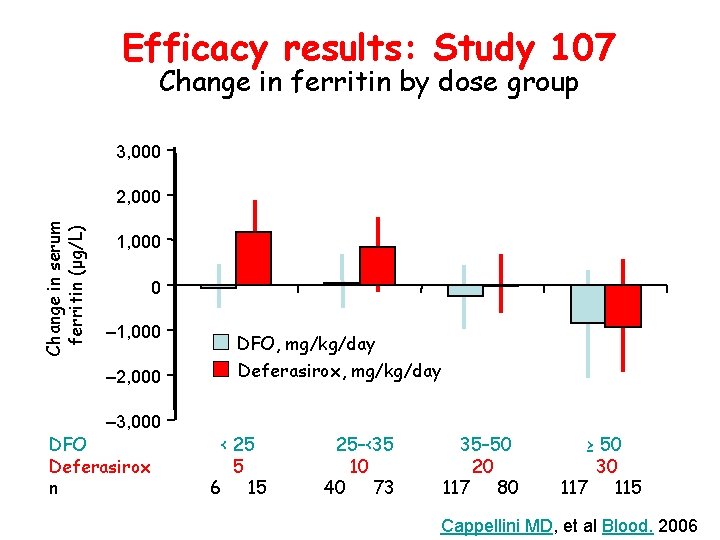
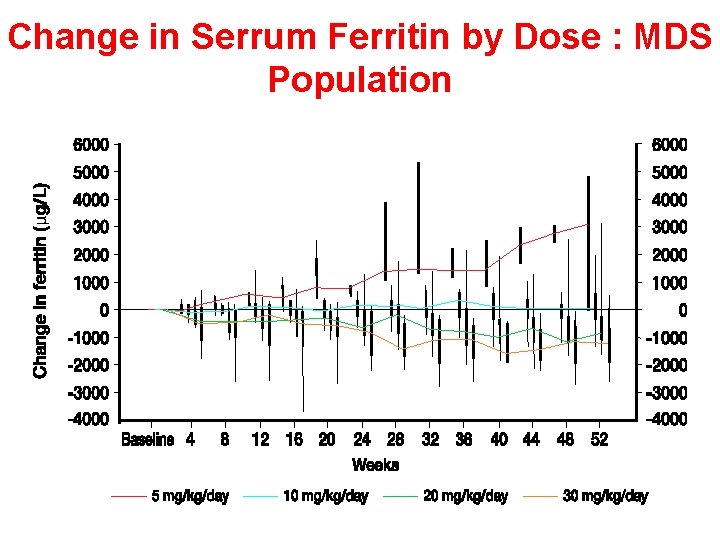
Efficacy results: Study 107 Change in ferritin by dose group 3, 000 Change in serum ferritin (μg/L) 2, 000 1, 000 0 – 1, 000 DFO, mg/kg/day – 2, 000 Deferasirox, mg/kg/day – 3, 000 DFO Deferasirox n < 25 5 6 15 25–<35 10 40 73 35– 50 20 117 80 ≥ 50 30 117 115 Cappellini MD, et al Blood. 2006

Study 0108

Study 0108: Phase II Single-Arm Trial Thalassemia and Other Anemias Study design – 1 -year trial • 85 patients with β thalassemia • 99 patients with rare anemia – Patients treated with deferasirox for 1 year – LIC assessed by liver biopsy or SQUID – Ongoing safety and serum ferritin monitoring

Disease # of patients Alpha Thal 1 Thal Intermedia 1 Fanconi's anemia 1 Chronic autoimmune hemolytic anemia 1 Congenital diserythropoietic anemia 1 Pyruvate kinase deficiency 2 Myelofibrosis 3 Pure Red Cell Aplasia 3 Aplastic anemia 4 Congenital Sideroblastic Anemia 5 unknown/Other 9 Diamond Blackfan MDS Beta Thal TOTAL 26 47 84 184

Change in LIC by Dose : Overall Population

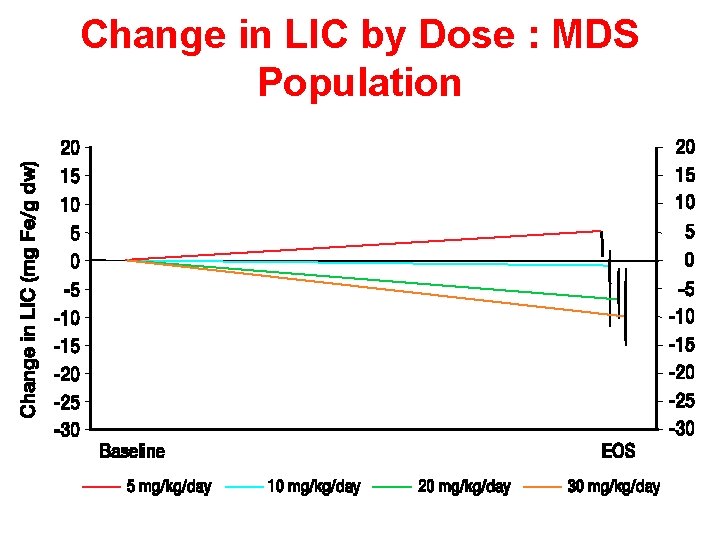
Change in LIC by Dose : MDS Population

Change in Serrum Ferritin by Dose : MDS Population

Deferasirox: Safety and Tolerability Profile • Most common adverse experiences in trials were nausea (10%), vomiting (9%), abdominal pain (14%), diarrhea (12%) and skin rash (8%) that rarely required discontinuation of study drug • Mild increases in creatinine occurred in some patients (3% exceeded ULN in thalassemia, 16% exceeded ULN in rare anemias) • No agranulocytosis observed in over 800 patients

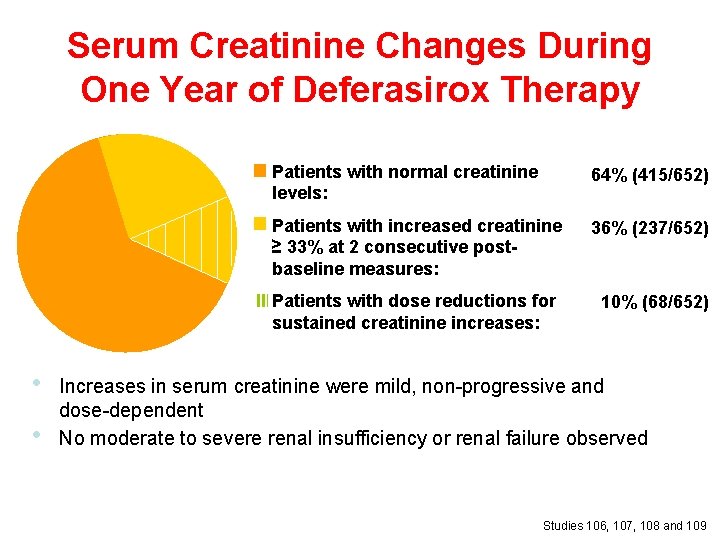
Serum Creatinine Changes During One Year of Deferasirox Therapy Patients with normal creatinine levels: 64% (415/652) Patients with increased creatinine ≥ 33% at 2 consecutive postbaseline measures: 36% (237/652) III Patients with dose reductions for sustained creatinine increases: • • 10% (68/652) Increases in serum creatinine were mild, non-progressive and dose-dependent No moderate to severe renal insufficiency or renal failure observed Studies 106, 107, 108 and 109

Results to Date • Literature documents the effects of iron overload on cardiac, hepatic and endocrine function in MDS • Small series indicate the benefits of chelation in MDS • Ph. II and III trials show that deferasirox produces statistically significant and clinically relevant reductions in LIC in Iron overloaded patients

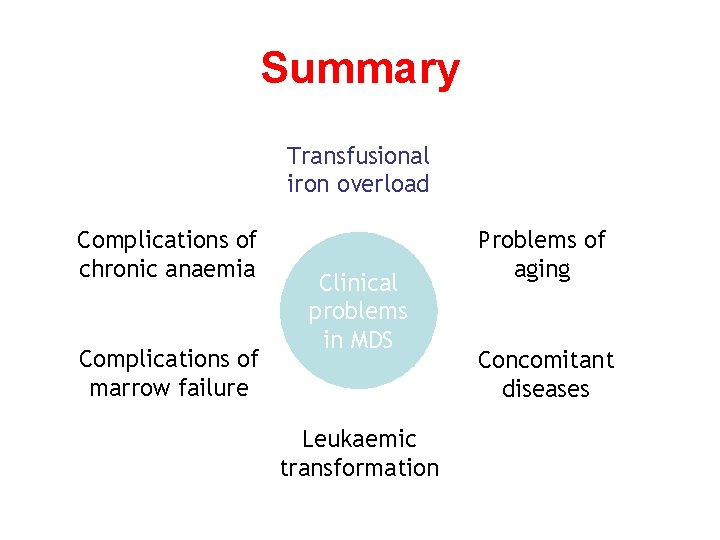
Summary Transfusional iron overload Complications of chronic anaemia Complications of marrow failure Clinical problems in MDS Leukaemic transformation Problems of aging Concomitant diseases

Summary • How much morbidity and mortality is attributable to iron overload? — Clinical studies — Thalassaemia experience — Prognostic assessment of MDS —“Expert opinions”/common sense
 What is an iron infusion
What is an iron infusion Determinazione del ferro con ortofenantrolina
Determinazione del ferro con ortofenantrolina Canzone dei diritti dei bambini
Canzone dei diritti dei bambini Quale sono i poligoni
Quale sono i poligoni Agnus dei qui tollis peccata mundi miserere nobis
Agnus dei qui tollis peccata mundi miserere nobis Luis felipe ferro
Luis felipe ferro Campuran antar besi(fe) dan karbon 4℅,disebut....
Campuran antar besi(fe) dan karbon 4℅,disebut.... Enrico ferro
Enrico ferro Recozimento
Recozimento Tania ferro
Tania ferro Ferro fundido fc 200
Ferro fundido fc 200 Punto eutettico ferro carbonio
Punto eutettico ferro carbonio Massimiliano ferro
Massimiliano ferro Omnibus ferro militis perterritis
Omnibus ferro militis perterritis Rnando
Rnando O prego de ferro ab inicialmente não imantado
O prego de ferro ab inicialmente não imantado Forno cubilô
Forno cubilô Um bloco metalico com 100g de massa a 225
Um bloco metalico com 100g de massa a 225 Para and ferro magnetism
Para and ferro magnetism Vunesp quando uma pessoa encosta a mão em um ferro quente
Vunesp quando uma pessoa encosta a mão em um ferro quente Ferro fundido
Ferro fundido Aço e ferro fundido
Aço e ferro fundido Una pallina di ferro da 30 g viene lasciata cadere
Una pallina di ferro da 30 g viene lasciata cadere Ferro fundido
Ferro fundido Para o elemento ferro (z=26) a alternativa verdadeira
Para o elemento ferro (z=26) a alternativa verdadeira Kaz ferro steel
Kaz ferro steel Ferro de passar
Ferro de passar Ossido di ferro marte
Ossido di ferro marte Ergo nomic
Ergo nomic Vicarius filii dei meaning
Vicarius filii dei meaning Lo statuto dei diritti del contribuente
Lo statuto dei diritti del contribuente Carta dei diritti del turista 2020
Carta dei diritti del turista 2020 Teoria del campo dei leganti
Teoria del campo dei leganti Unione dei comuni del frignano
Unione dei comuni del frignano Termini primitivi geometria
Termini primitivi geometria Poteri datore di lavoro
Poteri datore di lavoro Atteggiamento lordico
Atteggiamento lordico Modificazione dei soggetti del rapporto obbligatorio
Modificazione dei soggetti del rapporto obbligatorio Vnb reizen
Vnb reizen I regni dei viventi
I regni dei viventi Che cos'è la valutazione dei rischi
Che cos'è la valutazione dei rischi Matrici ecologiche e dei sostegni
Matrici ecologiche e dei sostegni Spinte metallostatiche
Spinte metallostatiche Caduta dei gravi zanichelli
Caduta dei gravi zanichelli Legge biot savart
Legge biot savart Esperimento di cavendish zanichelli
Esperimento di cavendish zanichelli Tesina sui diritti civili collegamenti
Tesina sui diritti civili collegamenti Membranofono sumero
Membranofono sumero Teorema del decentramento di oates
Teorema del decentramento di oates Ottimo paretiano teoria dei giochi
Ottimo paretiano teoria dei giochi