Midas Health Analytics Solutions An Overview of the































- Slides: 54

Midas Health Analytics Solutions An Overview of the CMS FY 2019 IPPS Final Rule Vance Newman Regulatory Product Manager Midas Annual Symposium | September 16 – 20, 2018 Westin La Paloma Resort & Spa Tucson, Arizona Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 1

Midas Health Analytics Solutions Additional information included in the slide notes. Midas Annual Symposium | September 16 – 20, 2018 Westin La Paloma Resort & Spa Tucson, Arizona Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 2

FY 2019 IPPS Final Rule Federal Register Review of FINAL IPPS Rule for FY 2019 CMS-1694 -F 42 CFR Parts 412, 413, 424, and 495 Published to Federal Registry Aug 17, 2018. Download the FY 2019 IPPS Final Rule from the Federal Register https: //www. federalregister. gov/d/2 018 -16766 Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 3

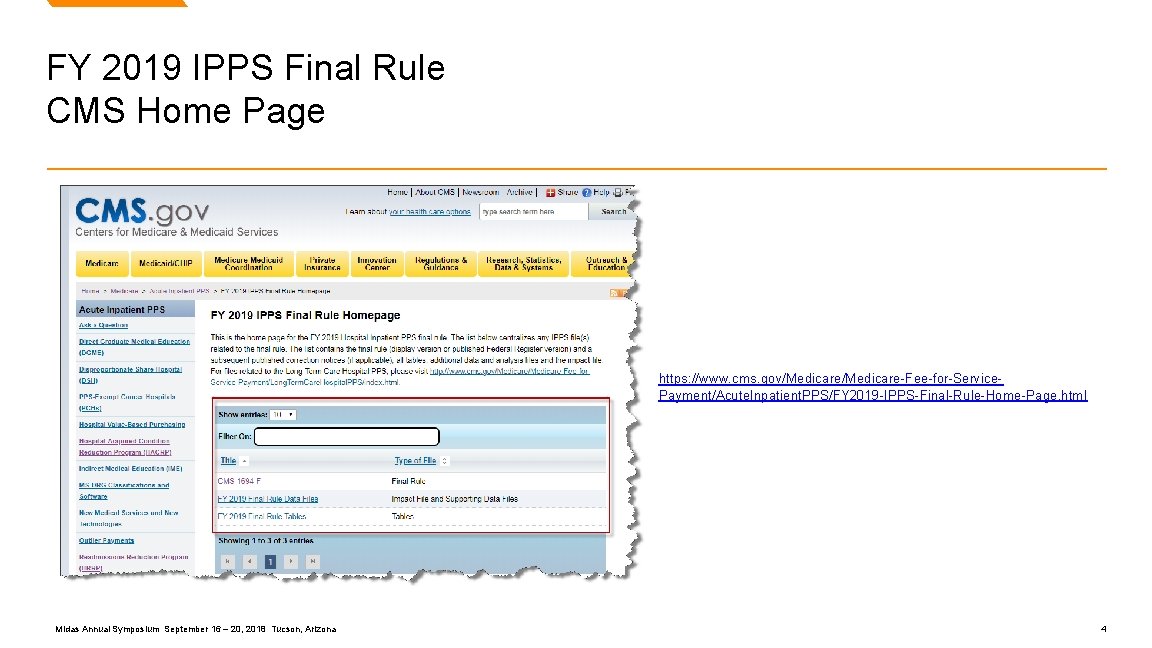
FY 2019 IPPS Final Rule CMS Home Page https: //www. cms. gov/Medicare-Fee-for-Service. Payment/Acute. Inpatient. PPS/FY 2019 -IPPS-Final-Rule-Home-Page. html Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 4

Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 5

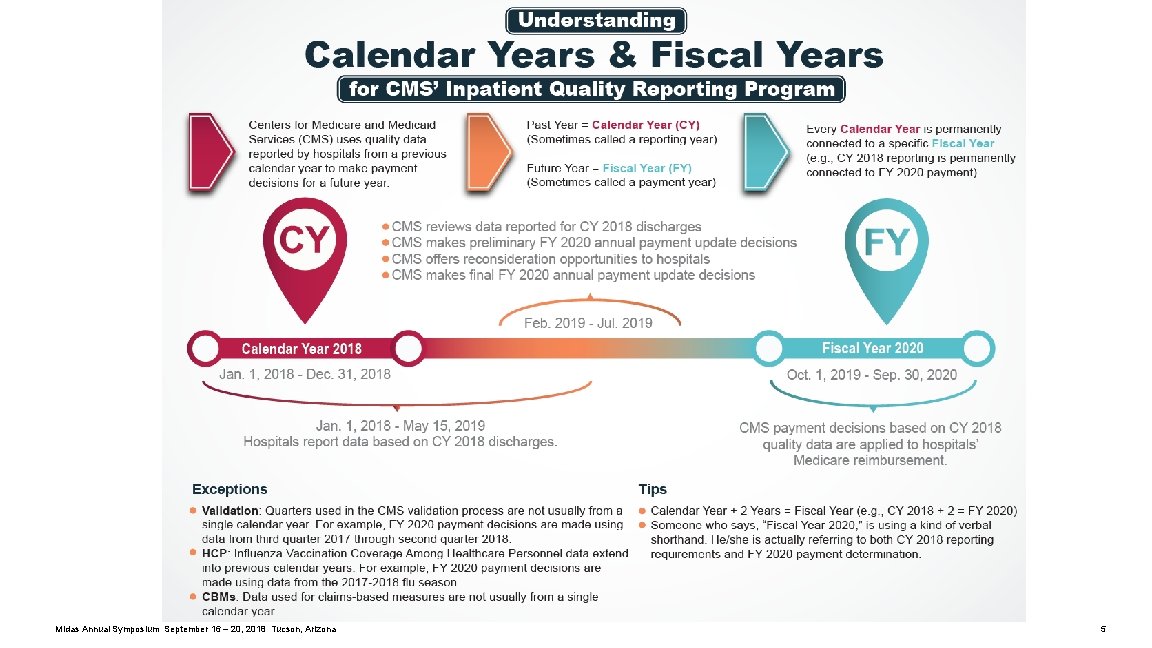
Resources Quality. Net Hospital Inpatient and Outpatient Quality Reporting Outreach and Education Support Centers Inpatient Quality Reporting Programs: https: //www. qualityreportingcenter. com/inpatient/ Upcoming Events: https: //www. qualityreportingcenter. com/inpatient/iqr/upcoming/ Archived Events: https: //www. qualityreportingcenter. com/inpatient/iqr/events/ Resources and Tools: https: //www. qualityreportingcenter. com/inpatient/iqr/resources-and-tools/ Hospital Inpatient Quality Reporting (IQR) Program: https: //www. qualitynet. org/dcs/Content. Server? c=Page&pagename=Qnet. Public%2 FPage%2 FQnet. Tier 2&cid=1138115987129 Understanding Calendar Years & Fiscal Years: https: //www. qualityreportingcenter. com/wp-content/uploads/2018/05/CY-FY_FINAL 508. pdf Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 6

Background on the IPPS and LTCH PPS Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 7

Background on the IPPS and LTCH PPS CMS pays acute care hospitals (with a few exceptions specified in the law) for inpatient stays under the IPPS and long-term care hospitals under the LTCH PPS. CMS sets base payment rates prospectively for inpatient stays based on the patient’s diagnosis and severity of illness. Subject to certain adjustments, a hospital receives a single payment for the case based on the payment classification – Medicare Severity Diagnosis-Related Groups (MS-DRGs) under the IPPS and Medicare Severity Long-Term Care Diagnosis-Related Groups (MS-LTC-DRGs) under the LTCH PPS – which are assigned at discharge. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 8

Background on the IPPS and LTCH PPS By law, CMS is required to update payment rates for IPPS hospitals annually, and to account for changes in the prices of goods and services used by these hospitals in treating Medicare patients, as well as for other factors. This is known as the hospital “market basket. ” The IPPS pays hospitals for services provided to Medicare beneficiaries using a national base payment rate, adjusted for a number of factors that affect each hospital’s costs, including the patient’s condition and the cost of hospital labor in the given hospital’s geographic area. Payment rates to LTCHs are typically updated annually according to a separate market based on LTCHspecific goods and services. The changes, which will affect approximately 3, 330 acute care hospitals and approximately 420 LTCHs, apply to discharges occurring on or after October 1, 2018. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 9

Interoperability Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 10

Request for Information on Interoperability CMS released a Request for Information in the IPPS/LTCH PPS proposed rule issued on April 24, 2018, to obtain feedback on positive solutions to better achieve interoperability, or the sharing of healthcare data between providers, which will inform next steps in advancing this critical initiative. In this final rule, CMS makes changes to the Promoting Interoperability Programs (formerly known as the EHR Incentive Programs) to increase interoperability and flexibility while reducing burden and placing a strong emphasis on measures that require the exchange of health information between providers and patients. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 11

Interoperability In 2011, the Medicare and Medicaid Promoting Interoperability Programs (known then as the Medicare and Medicaid EHR Incentive Programs) were established to encourage eligible professionals, eligible hospitals, and critical access hospitals (CAHs) to adopt, implement, upgrade (AIU), and demonstrate meaningful use of certified EHR technology (CEHRT). In this final rule, CMS overhauls the Medicare and Medicaid Promoting Interoperability Programs in order to better achieve program goals. Key provisions of this overhaul include the following: Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona • The rule finalizes an EHR reporting period of a minimum of any continuous 90 -day period in each of the calendar years (CYs) 2019 and 2020 for new and returning participants attesting to CMS or their State Medicaid agency. • CMS also reiterates that beginning with an EHR reporting period in CY 2019, all eligible hospitals and CAHs under the Medicare and Medicaid Promoting Interoperability Programs are required to use the 2015 Edition of CEHRT. • For the Medicare Promoting Interoperability Program, the rule finalizes a new performance-based scoring methodology consisting of a smaller set of objectives that will provide a more flexible, less-burdensome structure, allowing eligible hospitals and CAHs to place their focus back on patients. • CMS finalizes two new e-Prescribing measures related to e-prescribing of opioids (Schedule II controlled substances). The Query of PDMP measure will be optional in CY 2019 and will be required beginning in CY 2020. 12

Transparency Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 13

Transparency Online Posting of Standard Charges Under current law, hospitals are required to establish and make public a list of their standard charges. Effective CY 2019 CMS updated the guidelines to specifically require hospitals to make public a list of their standard charges via the Internet in a machine-readable format, and to update this information at least annually, or more often as appropriate. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 14

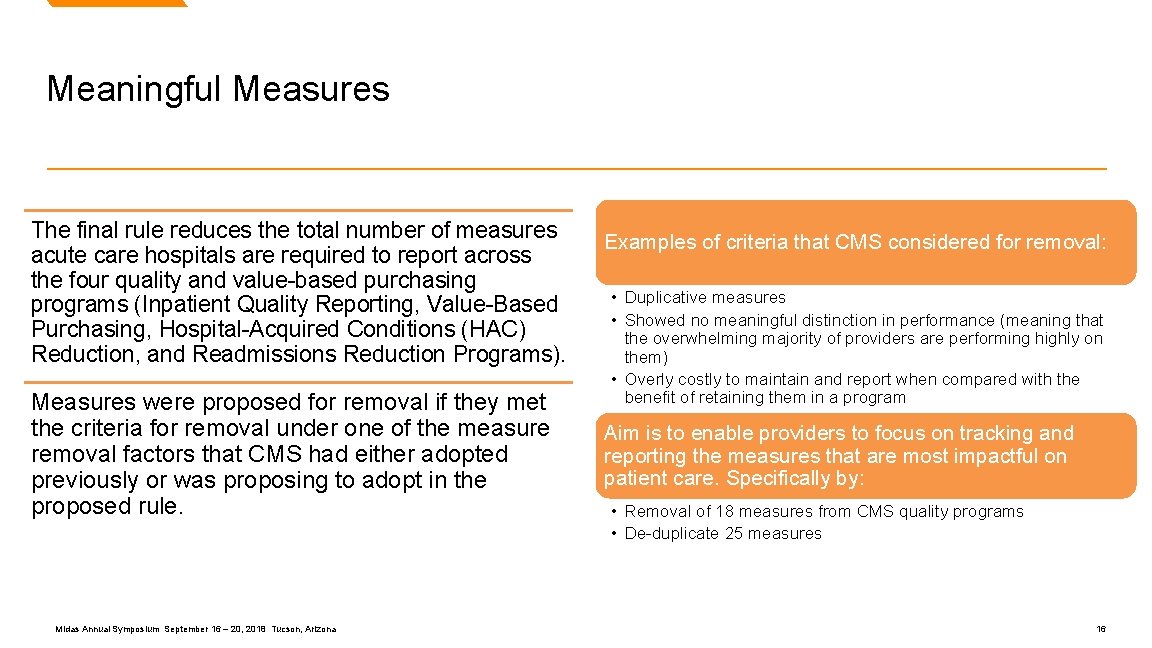
Meaningful Measures Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 15

Meaningful Measures The final rule reduces the total number of measures acute care hospitals are required to report across the four quality and value-based purchasing programs (Inpatient Quality Reporting, Value-Based Purchasing, Hospital-Acquired Conditions (HAC) Reduction, and Readmissions Reduction Programs). Measures were proposed for removal if they met the criteria for removal under one of the measure removal factors that CMS had either adopted previously or was proposing to adopt in the proposed rule. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona Examples of criteria that CMS considered for removal: • Duplicative measures • Showed no meaningful distinction in performance (meaning that the overwhelming majority of providers are performing highly on them) • Overly costly to maintain and report when compared with the benefit of retaining them in a program Aim is to enable providers to focus on tracking and reporting the measures that are most impactful on patient care. Specifically by: • Removal of 18 measures from CMS quality programs • De-duplicate 25 measures 16

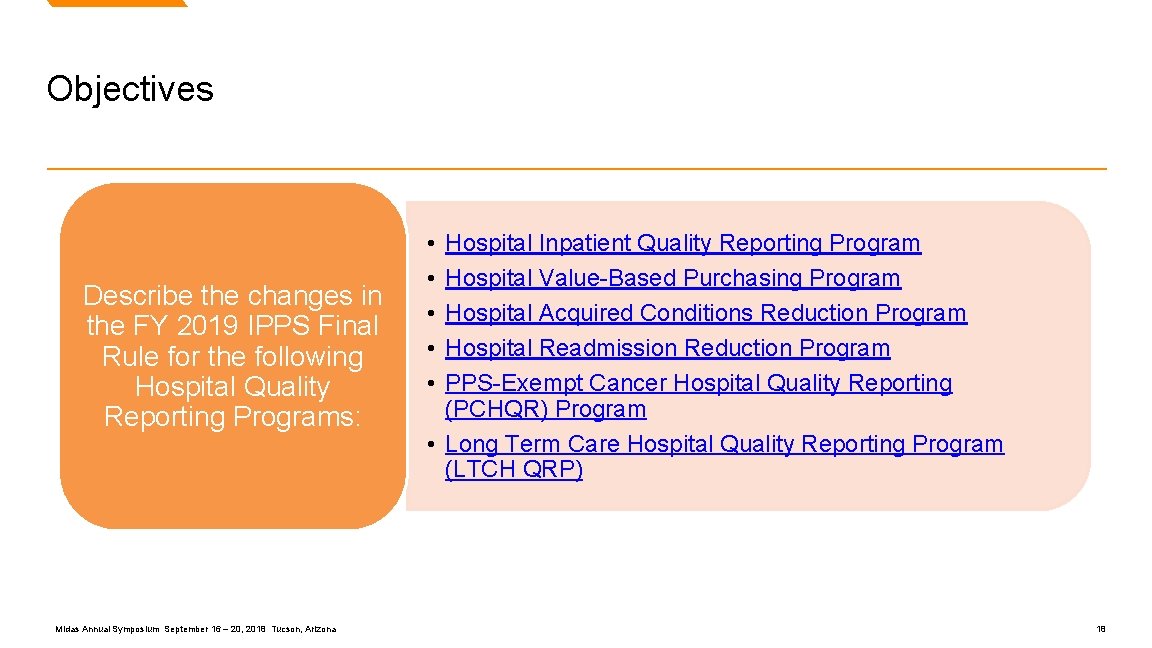
Objectives for IPPS Final Rule Review Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 17

Objectives Describe the changes in the FY 2019 IPPS Final Rule for the following Hospital Quality Reporting Programs: Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona • • • Hospital Inpatient Quality Reporting Program Hospital Value-Based Purchasing Program Hospital Acquired Conditions Reduction Program Hospital Readmission Reduction Program PPS-Exempt Cancer Hospital Quality Reporting (PCHQR) Program • Long Term Care Hospital Quality Reporting Program (LTCH QRP) 18

Hospital Inpatient Quality Reporting (IQR) Program Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 19

Hospital Inpatient Quality Reporting (IQR) Program – Begins pg. 41538 Summary • Remove 18 previously adopted measures that are “topped out, ” do not result in better patient outcomes, or have associated costs that outweigh the benefit of continued use in the program. • De-duplicate 21 measures to simplify and streamline measures across programs. These measures will remain in one of the other four hospital quality programs. • The six healthcare-associated infection (HAI) patient safety measures that are being de-duplicated will be removed for CY 2020, which is one year later than originally proposed. • Details about measures being removed and rationale are on the following slides. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 20

Overview of Hospital Inpatient Quality Reporting (IQR) Program Measure Removals Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 21

Quick Links to Sections Healthcare-Associated Infection Measures and Removal Rationale Patient Safety Measures and Removal Rationale Structural Measures and Removal Rationale Mortality Outcome Measures and Removal Rationale Coordination of Care Measures and Removal Rationale Resource Use/Payment Measures and Removal Rationale Clinical Process of Care Measures and Removal Rationale Electronic Clinical Quality Measures and Removal Rationale Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 22

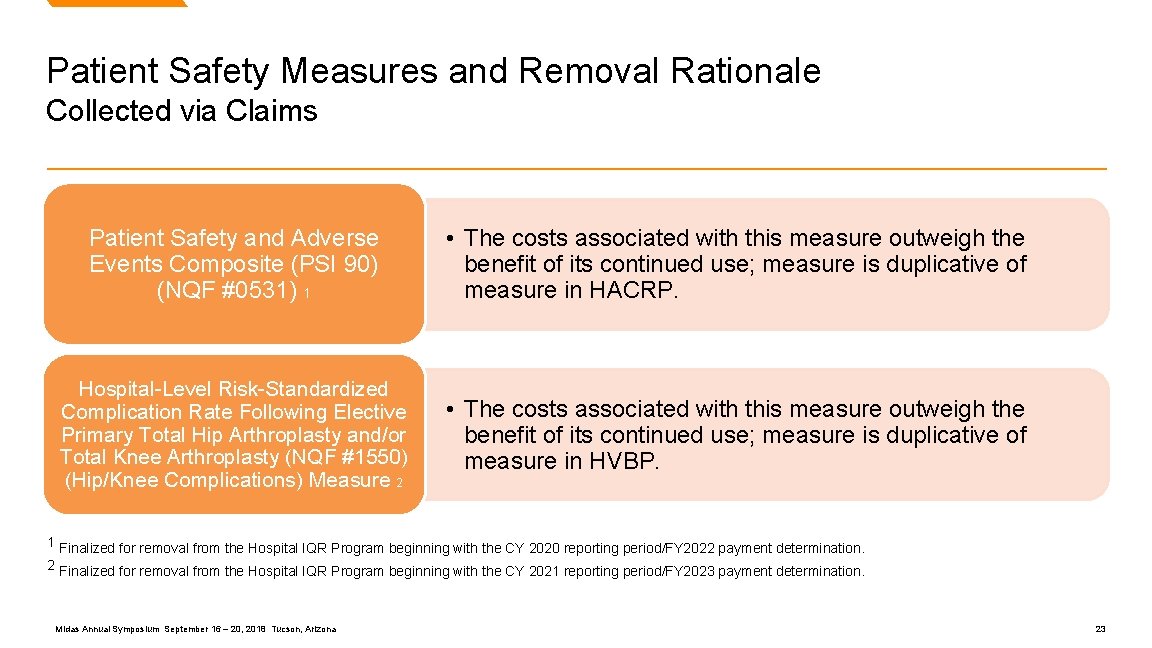
Patient Safety Measures and Removal Rationale Collected via Claims Patient Safety and Adverse Events Composite (PSI 90) (NQF #0531) 1 • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP. Hospital-Level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty and/or Total Knee Arthroplasty (NQF #1550) (Hip/Knee Complications) Measure 2 • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP. 1 Finalized for removal from the Hospital IQR Program beginning with the CY 2020 reporting period/FY 2022 payment determination. 2 Finalized for removal from the Hospital IQR Program beginning with the CY 2021 reporting period/FY 2023 payment determination. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 23

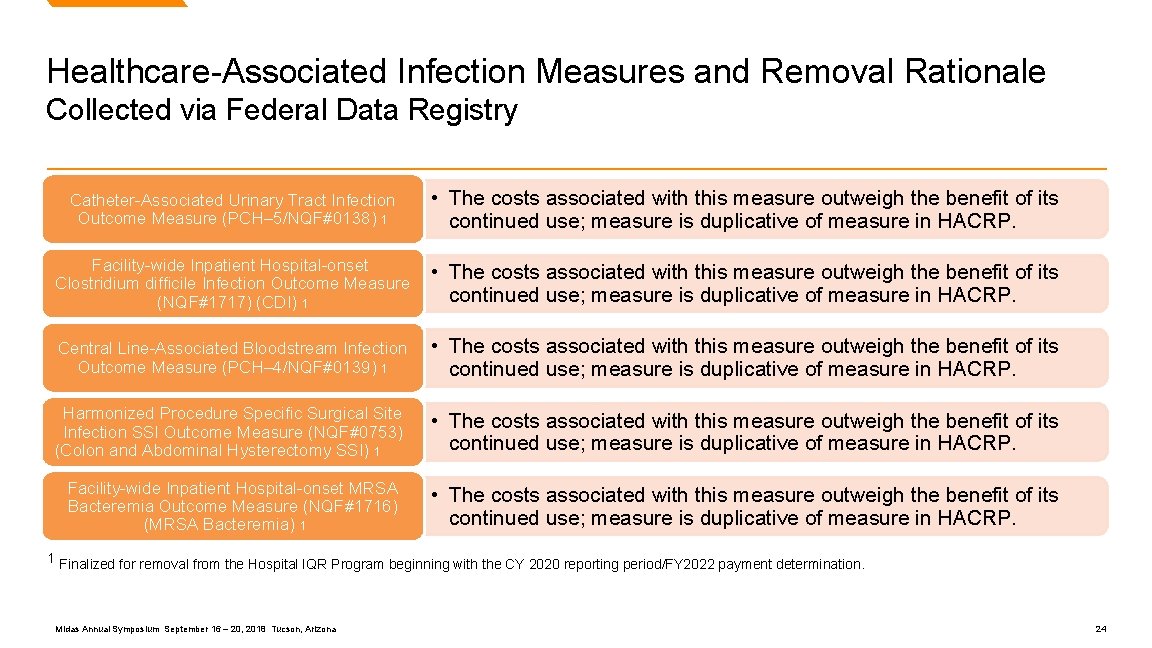
Healthcare-Associated Infection Measures and Removal Rationale Collected via Federal Data Registry Catheter-Associated Urinary Tract Infection Outcome Measure (PCH– 5/NQF#0138) 1 • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP. Facility-wide Inpatient Hospital-onset Clostridium difficile Infection Outcome Measure (NQF#1717) (CDI) 1 • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP. Central Line-Associated Bloodstream Infection Outcome Measure (PCH– 4/NQF#0139) 1 • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP. Harmonized Procedure Specific Surgical Site Infection SSI Outcome Measure (NQF#0753) (Colon and Abdominal Hysterectomy SSI) 1 • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP. Facility-wide Inpatient Hospital-onset MRSA Bacteremia Outcome Measure (NQF#1716) (MRSA Bacteremia) 1 • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP. 1 Finalized for removal from the Hospital IQR Program beginning with the CY 2020 reporting period/FY 2022 payment determination. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 24

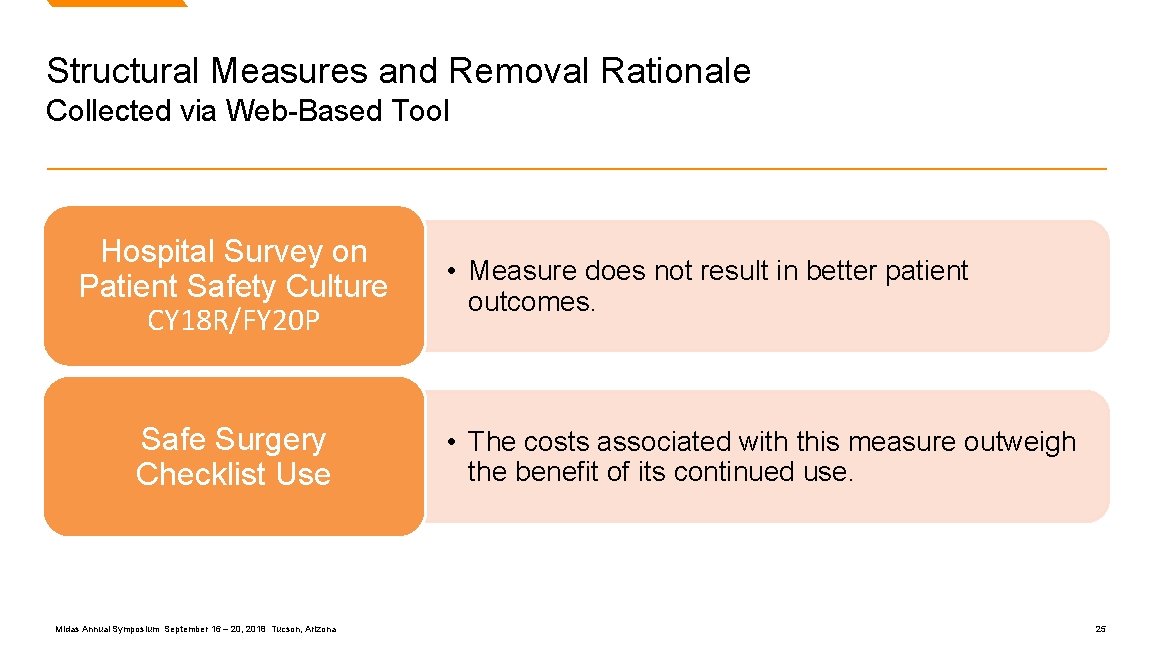
Structural Measures and Removal Rationale Collected via Web-Based Tool Hospital Survey on Patient Safety Culture CY 18 R/FY 20 P Safe Surgery Checklist Use Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona • Measure does not result in better patient outcomes. • The costs associated with this measure outweigh the benefit of its continued use. 25

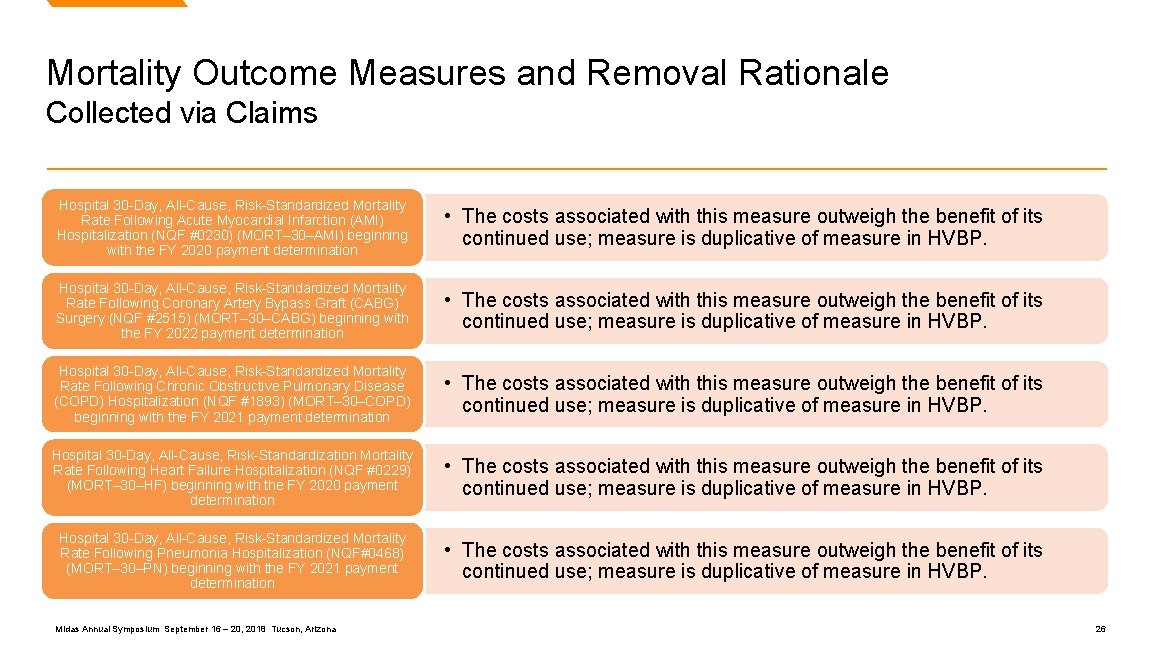
Mortality Outcome Measures and Removal Rationale Collected via Claims Hospital 30 -Day, All-Cause, Risk-Standardized Mortality Rate Following Acute Myocardial Infarction (AMI) Hospitalization (NQF #0230) (MORT– 30–AMI) beginning with the FY 2020 payment determination • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP. Hospital 30 -Day, All-Cause, Risk-Standardized Mortality Rate Following Coronary Artery Bypass Graft (CABG) Surgery (NQF #2515) (MORT– 30–CABG) beginning with the FY 2022 payment determination • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP. Hospital 30 -Day, All-Cause, Risk-Standardized Mortality Rate Following Chronic Obstructive Pulmonary Disease (COPD) Hospitalization (NQF #1893) (MORT– 30–COPD) beginning with the FY 2021 payment determination • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP. Hospital 30 -Day, All-Cause, Risk-Standardization Mortality Rate Following Heart Failure Hospitalization (NQF #0229) (MORT– 30–HF) beginning with the FY 2020 payment determination • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP. Hospital 30 -Day, All-Cause, Risk-Standardized Mortality Rate Following Pneumonia Hospitalization (NQF#0468) (MORT– 30–PN) beginning with the FY 2021 payment determination • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 26

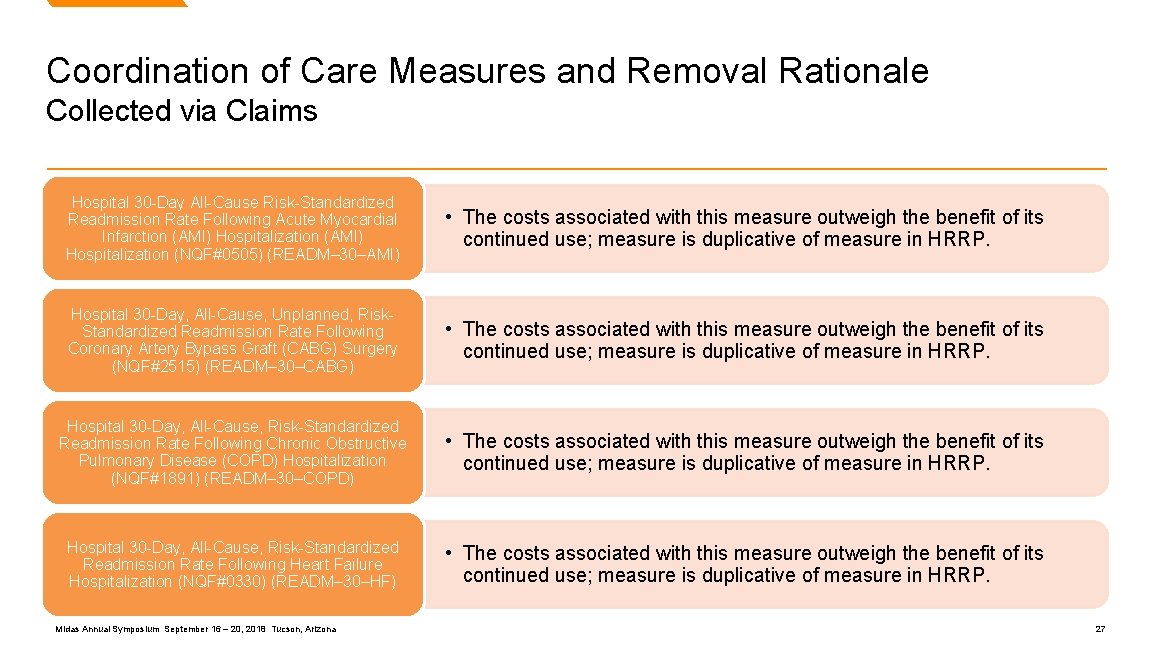
Coordination of Care Measures and Removal Rationale Collected via Claims Hospital 30 -Day All-Cause Risk-Standardized Readmission Rate Following Acute Myocardial Infarction (AMI) Hospitalization (NQF#0505) (READM– 30–AMI) • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HRRP. Hospital 30 -Day, All-Cause, Unplanned, Risk. Standardized Readmission Rate Following Coronary Artery Bypass Graft (CABG) Surgery (NQF#2515) (READM– 30–CABG) • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HRRP. Hospital 30 -Day, All-Cause, Risk-Standardized Readmission Rate Following Chronic Obstructive Pulmonary Disease (COPD) Hospitalization (NQF#1891) (READM– 30–COPD) • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HRRP. Hospital 30 -Day, All-Cause, Risk-Standardized Readmission Rate Following Heart Failure Hospitalization (NQF#0330) (READM– 30–HF) • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HRRP. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 27

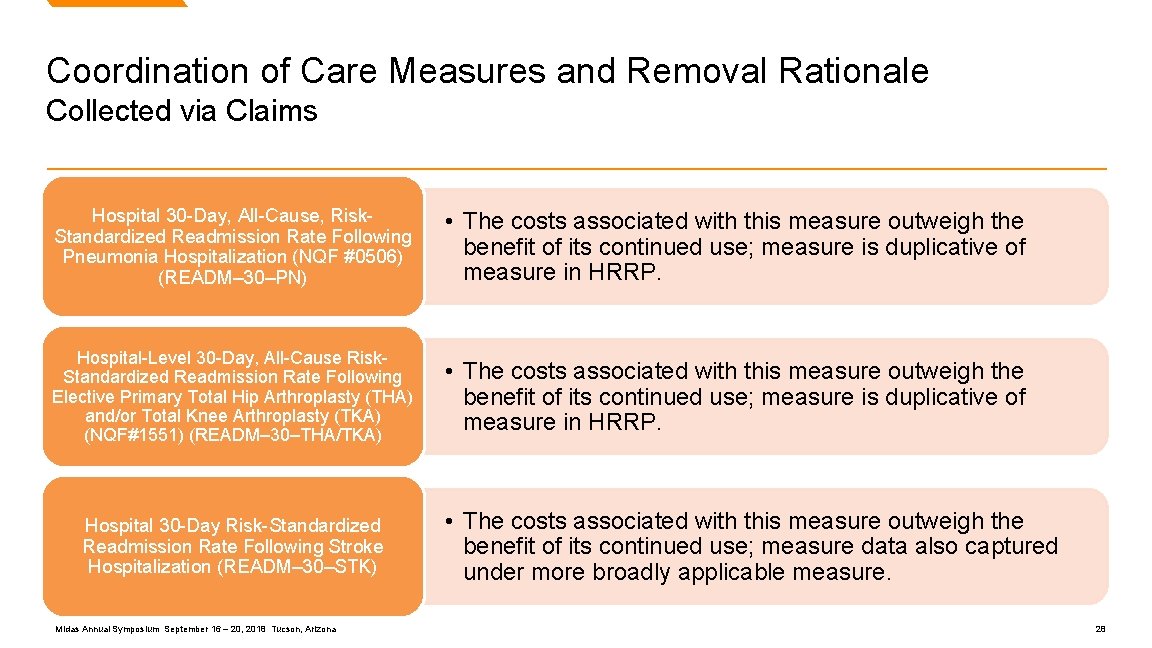
Coordination of Care Measures and Removal Rationale Collected via Claims Hospital 30 -Day, All-Cause, Risk. Standardized Readmission Rate Following Pneumonia Hospitalization (NQF #0506) (READM– 30–PN) • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HRRP. Hospital-Level 30 -Day, All-Cause Risk. Standardized Readmission Rate Following Elective Primary Total Hip Arthroplasty (THA) and/or Total Knee Arthroplasty (TKA) (NQF#1551) (READM– 30–THA/TKA) • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HRRP. Hospital 30 -Day Risk-Standardized Readmission Rate Following Stroke Hospitalization (READM– 30–STK) Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona • The costs associated with this measure outweigh the benefit of its continued use; measure data also captured under more broadly applicable measure. 28

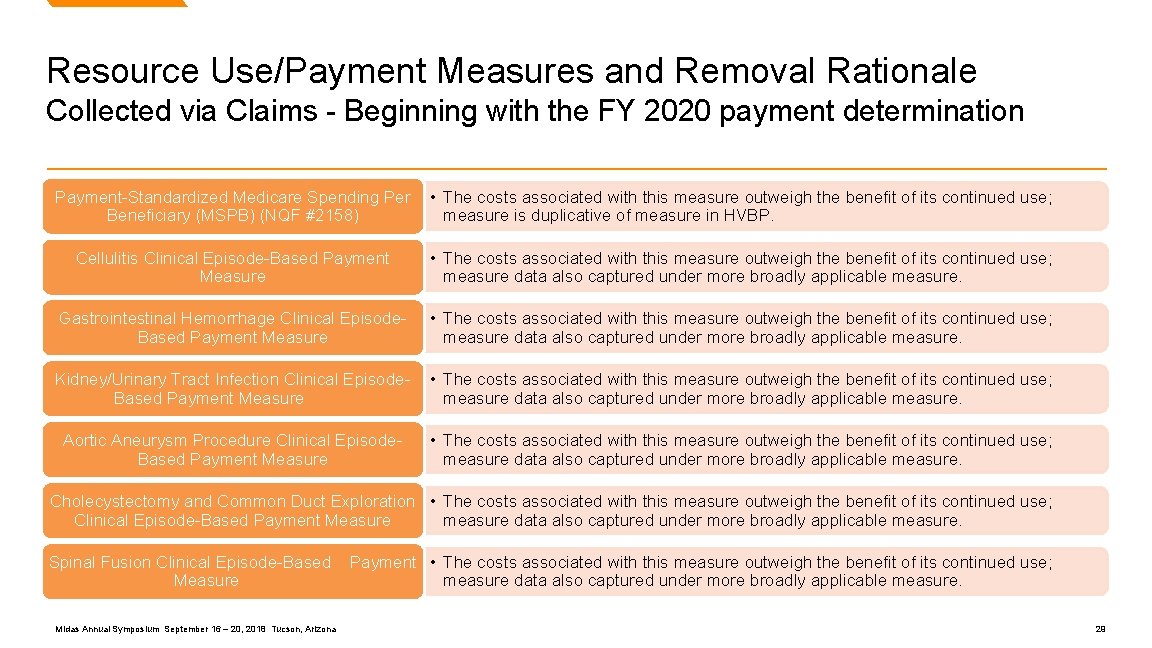
Resource Use/Payment Measures and Removal Rationale Collected via Claims - Beginning with the FY 2020 payment determination Payment-Standardized Medicare Spending Per Beneficiary (MSPB) (NQF #2158) • The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP. Cellulitis Clinical Episode-Based Payment Measure • The costs associated with this measure outweigh the benefit of its continued use; measure data also captured under more broadly applicable measure. Gastrointestinal Hemorrhage Clinical Episode. Based Payment Measure • The costs associated with this measure outweigh the benefit of its continued use; measure data also captured under more broadly applicable measure. Kidney/Urinary Tract Infection Clinical Episode. Based Payment Measure • The costs associated with this measure outweigh the benefit of its continued use; measure data also captured under more broadly applicable measure. Aortic Aneurysm Procedure Clinical Episode. Based Payment Measure • The costs associated with this measure outweigh the benefit of its continued use; measure data also captured under more broadly applicable measure. Cholecystectomy and Common Duct Exploration • The costs associated with this measure outweigh the benefit of its continued use; measure data also captured under more broadly applicable measure. Clinical Episode-Based Payment Measure Spinal Fusion Clinical Episode-Based Measure Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona Payment • The costs associated with this measure outweigh the benefit of its continued use; measure data also captured under more broadly applicable measure. 29

Clinical Process of Care Measures and Removal Rationale Collected via Chart Abstraction Median Time from ED Arrival to ED Departure for Admitted ED Patients (NQF #0495) (ED– 1) Admit Decision Time to ED Departure Time for Admitted Patients (NQF #0497) (ED– 2) • The costs associated with this measure outweigh the benefit of its continued use; e. CQM version of the measure will remain in the Hospital Inpatient Quality Reporting Program. Influenza Immunization (IMM– 2) • The costs associated with this measure outweigh the benefit of its continued use; measure performance is “topped-out. ” Incidence of Potentially Preventable Venous Thromboembolism Prophylaxis (VTE– 6) • The costs associated with this measure outweigh the benefit of its continued use. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 30

Electronic Clinical Quality Measures and Removal Rationale Collected via Electronic Health Record Beginning with the CY 2020 reporting period/FY 2022 payment determination Primary PCI Received Within 90 Minutes of • The costs associated with this measure outweigh the benefit of its continued use. Hospital Arrival (NQF#0163) (AMI– 8 a) Home Management Plan of Care Document Given to Patient/Caregiver (CAC– 3) • The costs associated with this measure outweigh the benefit of its continued use. Median Time from ED Arrival to ED Departure • The costs associated with this measure outweigh the benefit of its continued use. for Admitted ED Patients (NQF #0495) (ED– 1) Hearing Screening Prior to Hospital Discharge (NQF #1354) (EHDI– 1 a) • The costs associated with this measure outweigh the benefit of its continued use. Elective Delivery (NQF #0469) (PC– 01) • The costs associated with this measure outweigh the benefit of its continued use. Stroke Education (STK– 08) • The costs associated with this measure outweigh the benefit of its continued use. Assessed for Rehabilitation (NQF#0441) (STK– 10) • The costs associated with this measure outweigh the benefit of its continued use. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 31

Electronic Clinical Quality Measures Additional Information In addition, in alignment with the Promoting Interoperability Program, CMS finalizes two proposals in relation to the reporting of electronic clinical quality measures (e. CQMs) in the Hospital IQR Program beginning with the CY 2019 reporting period/FY 2021 payment determination: • Require that hospitals submit one, self-selected calendar quarter of discharge data for 4 e. CQMs in the Hospital IQR Program measure set, which is a continuation of the same reporting requirements previously adopted for the CY 2018 reporting period/FY 2020 payment determination; and • Require the use of the 2015 Edition of Certified Electronic Health Record Technology for e. CQMs. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 32

Hospital Value-Based Purchasing (VBP) Program Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 33

Hospital Value-Based Purchasing (VBP) Program - Begins pg. 41440 CMS finalized updates to the Hospital VBP Program include the removal of four duplicative measures, all of which are also included in the Hospital IQR Program measure set. CMS is not finalizing its proposals to remove six patient safety measures that are also in the Hospital-Acquired Condition Reduction Program measure set due to their critical importance to quality improvement and patient safety in the hospital setting and to strongly incentivize hospitals to continually strive for both improvement and high performance on these measures. • National Healthcare Safety Network (NHSN) Catheter- Associated Urinary Tract Infection (CAUTI) Outcome Measure (NQF#0138) • National Healthcare Safety Network (NHSN) Central Line-Associated Bloodstream Infection (CLABSI) Outcome Measure (NQF#0139) • American College of Surgeons-Centers for Disease Control and Prevention (ACS-CDC) Harmonized Procedure Specific Surgical Site Infection (SSI) Outcome Measure (NQF#0753) • National Healthcare Safety Network (NHSN) Facility-wide Inpatient Hospital-onset Methicillin-resistant Staphylococcus aureus Bacteremia (MRSA) Outcome Measure (NQF#1716) • National Healthcare Safety Network (NHSN) Facility-wide Inpatient Hospital-onset Clostridium difficile Infection (CDI) Outcome Measure (NQF#1717) • Patient Safety and Adverse Events (NQF#0531) (PSI 90) CMS is also not finalizing removal of the safety domain or revised weighting of the Hospital VBP Program domains. These policies are consistent with CMS’s commitment to patient safety as well as producing a smaller set of more meaningful measures and focusing on patient-centered outcomes. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 34

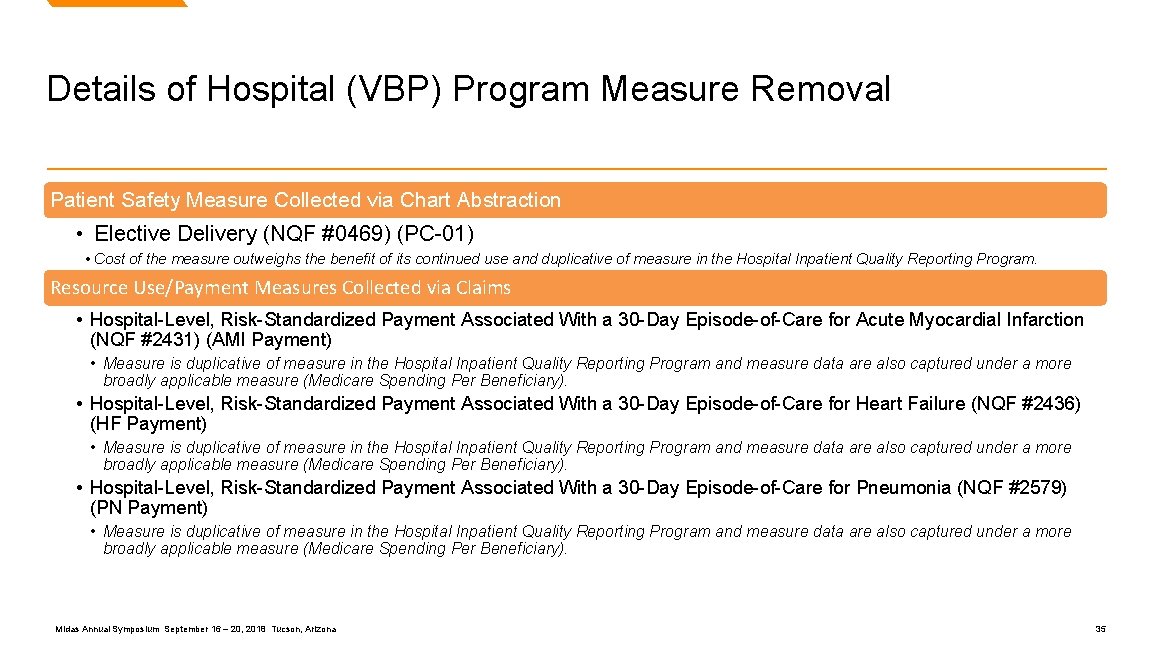
Details of Hospital (VBP) Program Measure Removal Patient Safety Measure Collected via Chart Abstraction • Elective Delivery (NQF #0469) (PC-01) • Cost of the measure outweighs the benefit of its continued use and duplicative of measure in the Hospital Inpatient Quality Reporting Program. Resource Use/Payment Measures Collected via Claims • Hospital-Level, Risk-Standardized Payment Associated With a 30 -Day Episode-of-Care for Acute Myocardial Infarction (NQF #2431) (AMI Payment) • Measure is duplicative of measure in the Hospital Inpatient Quality Reporting Program and measure data are also captured under a more broadly applicable measure (Medicare Spending Per Beneficiary). • Hospital-Level, Risk-Standardized Payment Associated With a 30 -Day Episode-of-Care for Heart Failure (NQF #2436) (HF Payment) • Measure is duplicative of measure in the Hospital Inpatient Quality Reporting Program and measure data are also captured under a more broadly applicable measure (Medicare Spending Per Beneficiary). • Hospital-Level, Risk-Standardized Payment Associated With a 30 -Day Episode-of-Care for Pneumonia (NQF #2579) (PN Payment) • Measure is duplicative of measure in the Hospital Inpatient Quality Reporting Program and measure data are also captured under a more broadly applicable measure (Medicare Spending Per Beneficiary). Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 35

Hospital-Acquired Conditions (HAC) Reduction Program Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 36

Hospital-Acquired Conditions (HAC) Reduction Program – Begins pg. 41472 In the FY 2019 IPPS/LTCH PPS final rule, CMS finalizes three changes to existing HAC Reduction Program policies. CMS finalizes the following policies to: • Specify the dates of the time period used to calculate hospital performance for the FY 2021 HAC Reduction Program • Adopt administrative processes to receive and validate National Healthcare Safety Network (NHSN) Healthcareassociated Infection (HAI) data that is submitted by hospitals to the Centers for Disease Control and Prevention (CDC) beginning CY 2020 • Adopt a new scoring methodology by removing domains and assigning equal weighting to each measure for which a hospital has a measure score in order to improve fairness across hospital types in the Program. Measures under the HAC Reduction Program will stay the same. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 37

Hospital Readmissions Reduction Program (HRRP) Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 38

Hospital Readmissions Reduction Program (HRRP) Begins pg. 41431 In the FY 2019 IPPS/LTCH PPS final rule, CMS finalized proposals to: • Establish the applicable period for the FY 2019, FY 2020, and FY 2021 program years. • Codify our previously finalized definitions of dual-eligible patients, proportion of dual-eligibles, and applicable period for dual-eligibility at 42 CFR 412. 152. In addition, CMS specifies the methodology for calculating aggregate payments for excess readmissions for FY 2019. Measures under the HRRP will stay the same. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 39

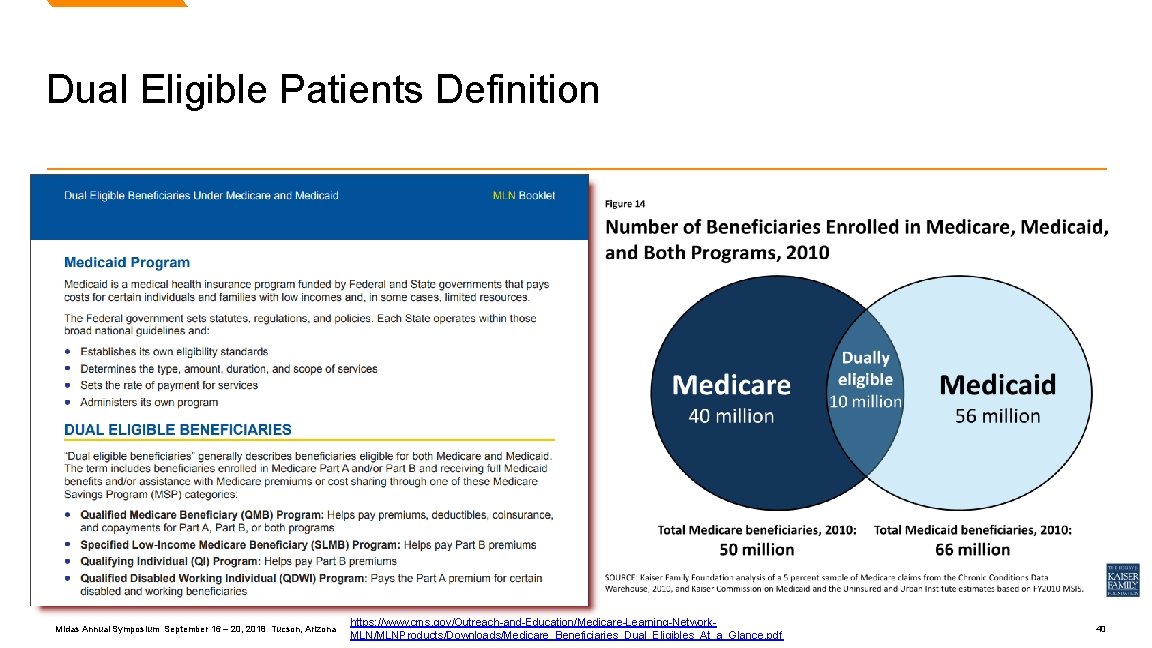
Dual Eligible Patients Definition Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona https: //www. cms. gov/Outreach-and-Education/Medicare-Learning-Network. MLN/MLNProducts/Downloads/Medicare_Beneficiaries_Dual_Eligibles_At_a_Glance. pdf 40

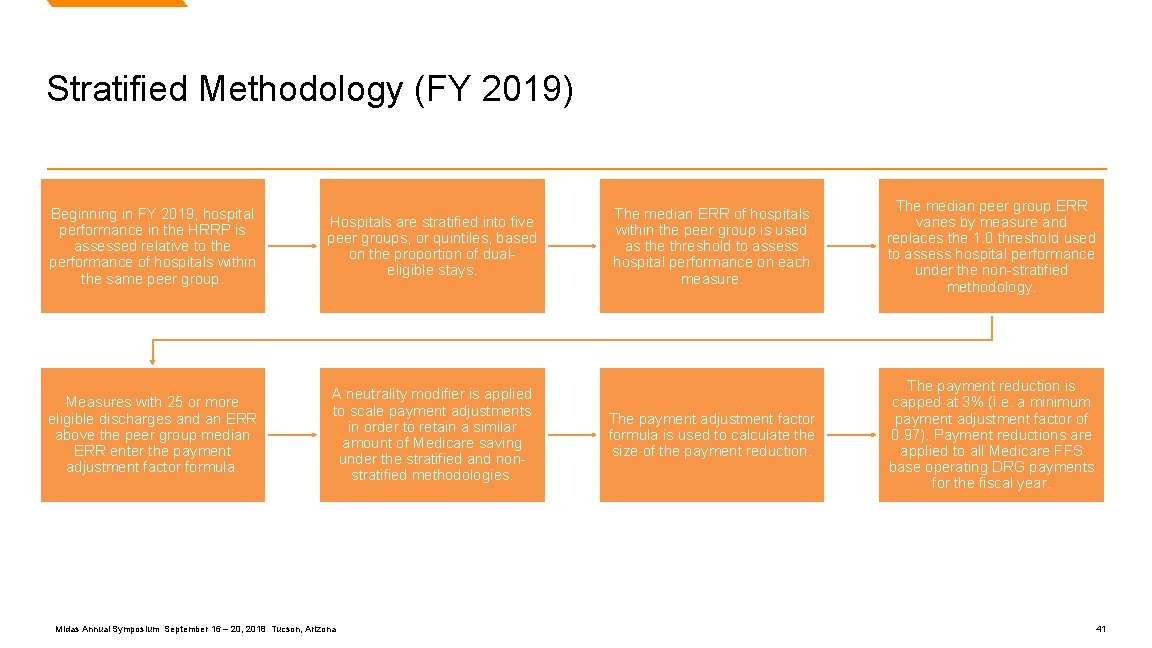
Stratified Methodology (FY 2019) Beginning in FY 2019, hospital performance in the HRRP is assessed relative to the performance of hospitals within the same peer group. Hospitals are stratified into five peer groups, or quintiles, based on the proportion of dualeligible stays. Measures with 25 or more eligible discharges and an ERR above the peer group median ERR enter the payment adjustment factor formula. A neutrality modifier is applied to scale payment adjustments in order to retain a similar amount of Medicare saving under the stratified and nonstratified methodologies. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona The median ERR of hospitals within the peer group is used as the threshold to assess hospital performance on each measure. The median peer group ERR varies by measure and replaces the 1. 0 threshold used to assess hospital performance under the non-stratified methodology. The payment adjustment factor formula is used to calculate the size of the payment reduction. The payment reduction is capped at 3% (i. e. a minimum payment adjustment factor of 0. 97). Payment reductions are applied to all Medicare FFS base operating DRG payments for the fiscal year. 41

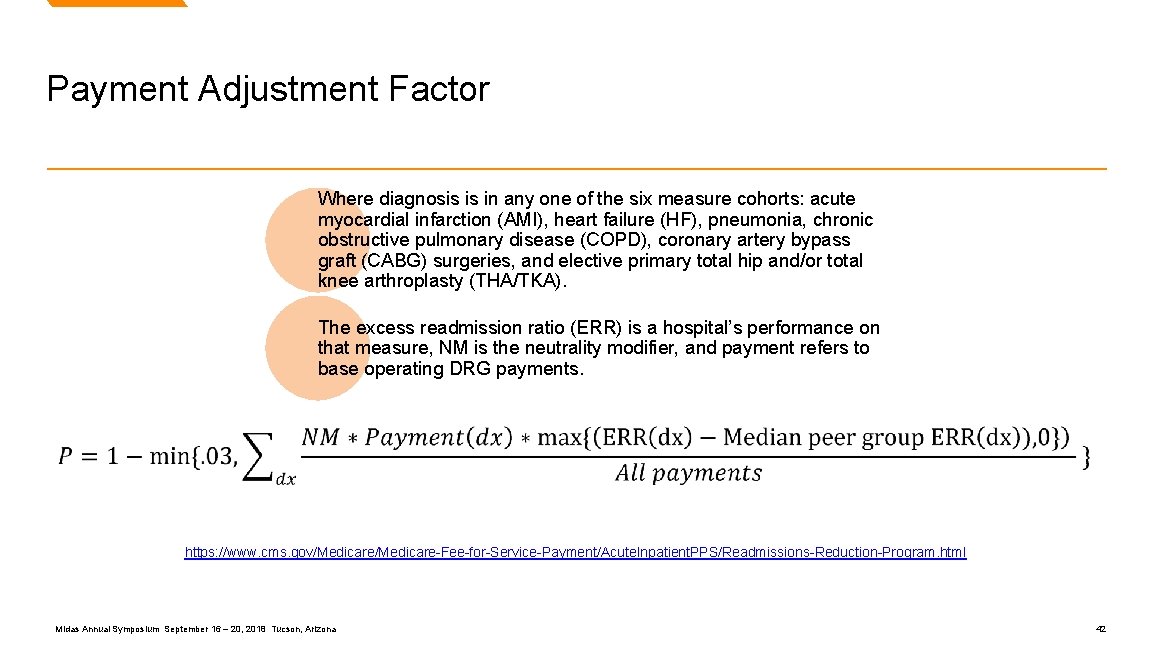
Payment Adjustment Factor Where diagnosis is in any one of the six measure cohorts: acute myocardial infarction (AMI), heart failure (HF), pneumonia, chronic obstructive pulmonary disease (COPD), coronary artery bypass graft (CABG) surgeries, and elective primary total hip and/or total knee arthroplasty (THA/TKA). The excess readmission ratio (ERR) is a hospital’s performance on that measure, NM is the neutrality modifier, and payment refers to base operating DRG payments. https: //www. cms. gov/Medicare-Fee-for-Service-Payment/Acute. Inpatient. PPS/Readmissions-Reduction-Program. html Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 42

PPS-Exempt Cancer Hospital Quality Reporting (PCHQR) Program Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 43

PPS-Exempt Cancer Hospital Quality Reporting (PCHQR) Program CMS finalized the following proposals: • Adoption of one new claims-based outcome measure beginning with the CY 2019 reporting period, Proportion of 30 -Day Unplanned Readmissions for Cancer Patients measure (NQF #3188); • Removal of four measures based on measure performance, beginning with the CY 2019 reporting period: • Oncology: Radiation Dose Limits to Normal Tissues (PCH– 14/NQF#0382) • Oncology: Medical and Radiation – Pain Intensity Quantified (PCH– 16/NQF #0384) • Prostate Cancer: Adjuvant Hormonal Therapy for High Risk Prostate Cancer Patients (PCH– 17/NQF#0390) • Prostate Cancer: Avoidance of Overuse of Bone Scan for Staging Low Risk Prostate Cancer Patients (PCH– 18/NQF #0389) • Adoption of one additional factor to consider when evaluating potential measures for removal from the PCHQR Program measure set, “The cost associated with the measure outweighs the benefit of its continued use in the program. ” Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 44

Details of (PCHQR) Program Measure Removal Structural Measures Collected via Web-based Tool Oncology: Radiation Dose Limits to Normal Tissues • Measure performance is “topped-out. ” Oncology: Medical and Radiation – Pain Intensity Quantified • Measure performance is “topped-out. ” Prostate Cancer: Adjuvant Hormonal Therapy for High Risk Patients • Measure performance is “topped-out. ” Prostate Cancer: Avoidance of Overuse of Bone Scan for Staging Low-Risk Patients • Measure performance is “topped-out. ” Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 45

Long Term Care Hospital Quality Reporting Program (LTCH QRP) Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 46

Long Term Care Hospital Quality Reporting Program (LTCH QRP) CMS finalized the following proposals; the final rule removes measures that either have significant operational challenges with reporting or are duplicative of other measures in the program. • National Healthcare Safety Network (NHSN) Facility-wide Inpatient Hospital-onset Methicillin-resistant Staphylococcus Aureus (MRSA) Bacteremia Outcome Measure (NQF #1716) (beginning with the FY 2020 LTCH QRP) • National Healthcare Safety Network (NHSN) Ventilator-Associated Event (VAE) Outcome Measure (beginning with the FY 2020 LTCH QRP • Percent of Residents or Patients Who Were Assessed and Appropriately Given the Seasonal Influenza Vaccine (Short Stay) (NQF #0680) (beginning with the FY 2021 LTCH QRP) Further, CMS finalized the following: • An update to the methods by which LTCHs are notified of non-compliance with the requirements of the LTCH QRP • An additional measure removal factor—the costs associated with a measure outweigh the benefit of its continued use in the program Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 47

Changes to Payment Rates Under IPPS Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 48

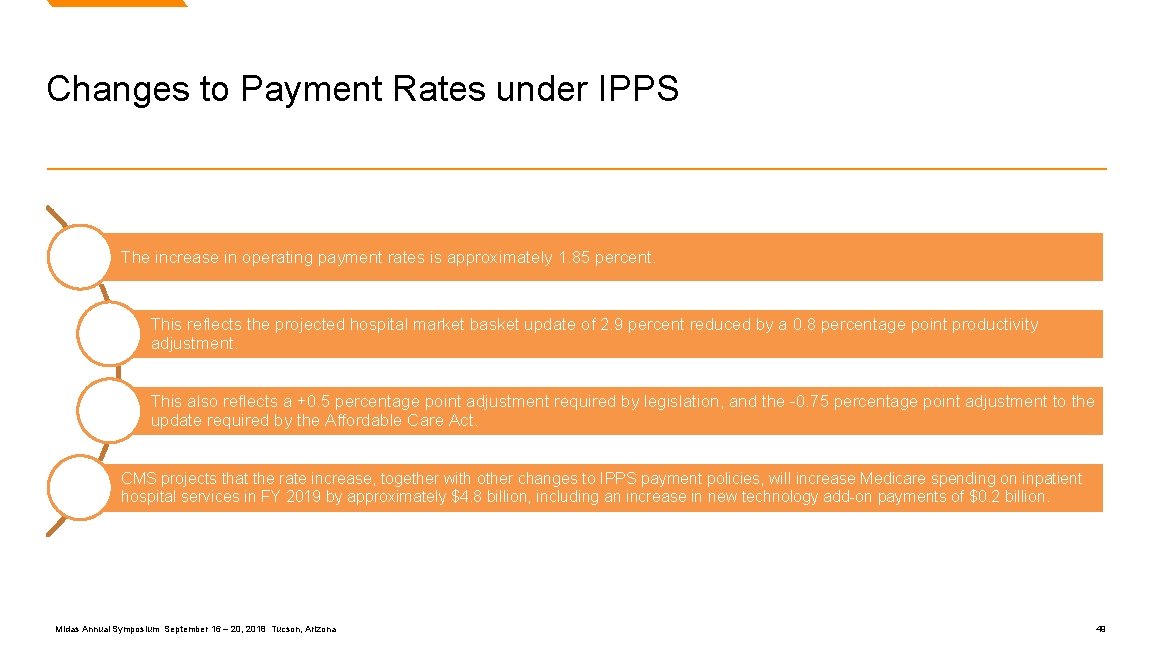
Changes to Payment Rates under IPPS The increase in operating payment rates is approximately 1. 85 percent. This reflects the projected hospital market basket update of 2. 9 percent reduced by a 0. 8 percentage point productivity adjustment. This also reflects a +0. 5 percentage point adjustment required by legislation, and the -0. 75 percentage point adjustment to the update required by the Affordable Care Act. CMS projects that the rate increase, together with other changes to IPPS payment policies, will increase Medicare spending on inpatient hospital services in FY 2019 by approximately $4. 8 billion, including an increase in new technology add-on payments of $0. 2 billion. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 49

Market Basket Update FY Market Basket % Multifactor Affordable Care Act Productivity Payment Reductions Adjustment Documentation and Coding Total Update % 2016 2. 4 -0. 2 -0. 5 -0. 8 0. 9 2017 2. 7 -0. 75 -0. 3 -1. 5 0. 15 2018 2. 7 -0. 75 -0. 6 0. 4588 1. 8088 2019 2. 9 -0. 75 -0. 8 0. 5 1. 85 Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 50

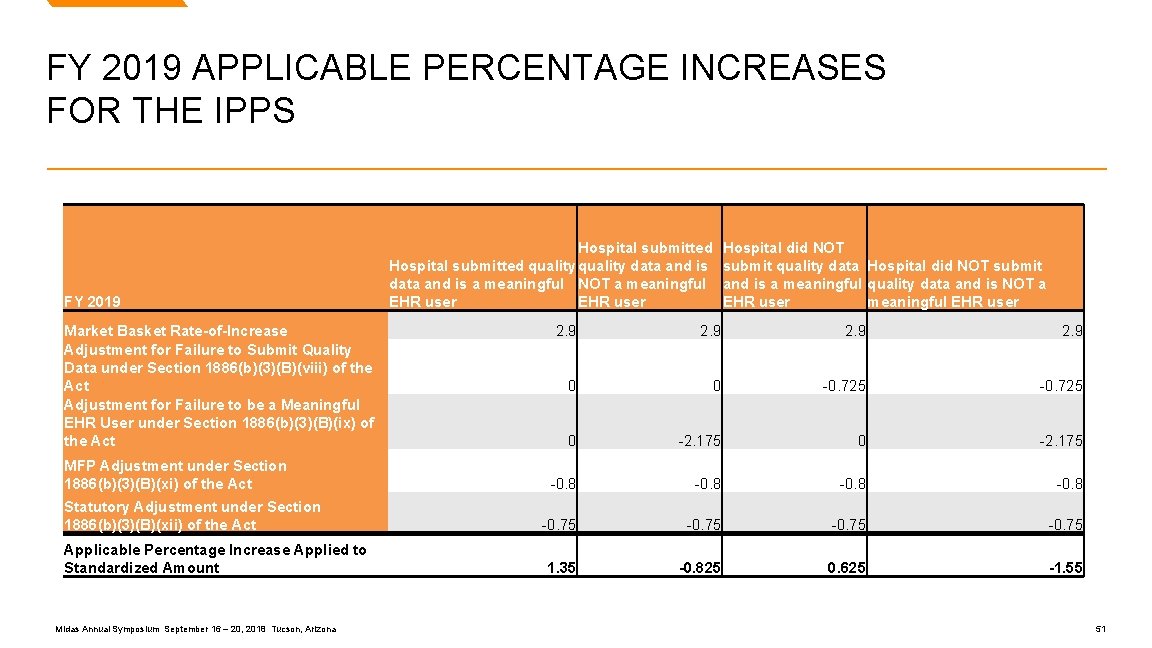
FY 2019 APPLICABLE PERCENTAGE INCREASES FOR THE IPPS FY 2019 Market Basket Rate-of-Increase Adjustment for Failure to Submit Quality Data under Section 1886(b)(3)(B)(viii) of the Act Adjustment for Failure to be a Meaningful EHR User under Section 1886(b)(3)(B)(ix) of the Act MFP Adjustment under Section 1886(b)(3)(B)(xi) of the Act Statutory Adjustment under Section 1886(b)(3)(B)(xii) of the Act Applicable Percentage Increase Applied to Standardized Amount Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona Hospital submitted quality data and is a meaningful NOT a meaningful EHR user Hospital did NOT submit quality data Hospital did NOT submit and is a meaningful quality data and is NOT a EHR user meaningful EHR user 2. 9 0 0 -0. 725 0 -2. 175 -0. 8 -0. 75 1. 35 -0. 825 0. 625 -1. 55 51

LTCH PPS Changes Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 52

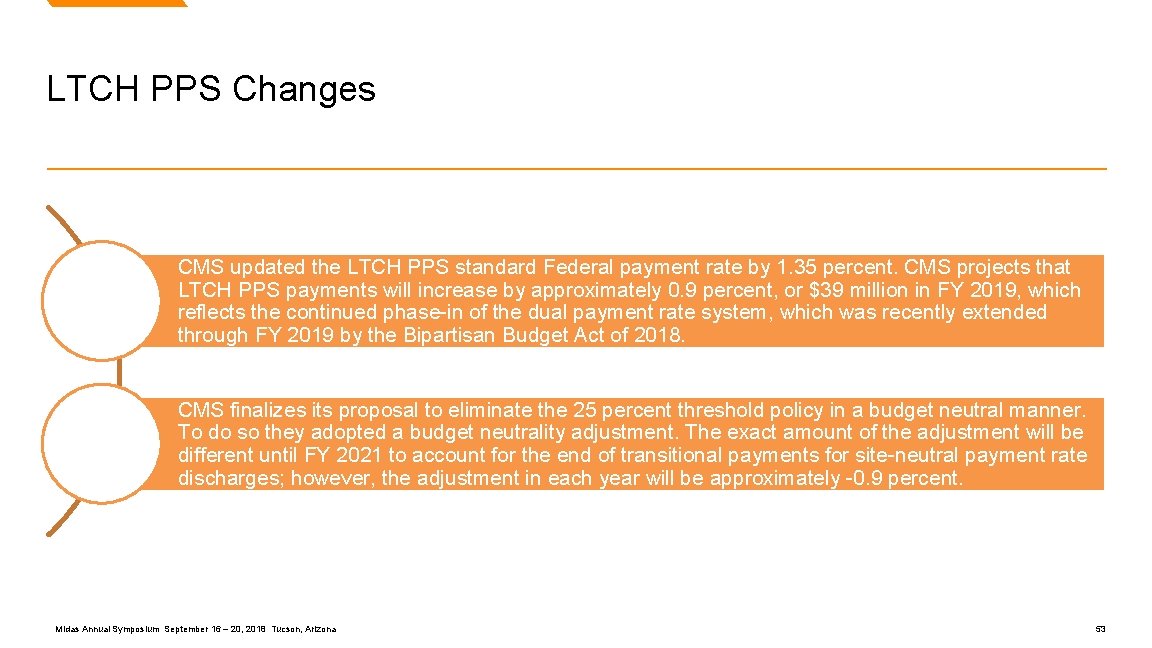
LTCH PPS Changes CMS updated the LTCH PPS standard Federal payment rate by 1. 35 percent. CMS projects that LTCH PPS payments will increase by approximately 0. 9 percent, or $39 million in FY 2019, which reflects the continued phase-in of the dual payment rate system, which was recently extended through FY 2019 by the Bipartisan Budget Act of 2018. CMS finalizes its proposal to eliminate the 25 percent threshold policy in a budget neutral manner. To do so they adopted a budget neutrality adjustment. The exact amount of the adjustment will be different until FY 2021 to account for the end of transitional payments for site-neutral payment rate discharges; however, the adjustment in each year will be approximately -0. 9 percent. Midas Annual Symposium September 16 – 20, 2018 Tucson, Arizona 53

Midas Health Analytics Solutions Thanks for attending! Any Questions? Vance Newman Regulatory Product Manager vance. newman@Conduent. com Midas Annual Symposium | September 16 – 20, 2018 Westin La Paloma Resort & Spa Tucson, Arizona © 2018 Conduent Business Service, LLC. All rights reserved. Conduent Agile. Arizona Star are trademarks of Conduent Business Services, LLC in the United States and/or other countries. Midas Annual Symposium September 16 – 20, and 2018 Tucson, 54
 Midas healthcare solutions inc
Midas healthcare solutions inc Teramond
Teramond Market overview managed file transfer solutions
Market overview managed file transfer solutions Example of nursing process
Example of nursing process Truven health micromedex
Truven health micromedex Predictive analytics in health insurance
Predictive analytics in health insurance Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton
Tư thế worm breton Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Các số nguyên tố là gì
Các số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Mrs midas 10 mark question
Mrs midas 10 mark question What does midas touch mean
What does midas touch mean Midas menarche interval duration
Midas menarche interval duration The legend of king midas
The legend of king midas Morale du mythe de midas
Morale du mythe de midas Mrs midas
Mrs midas Miss midas
Miss midas













































































