Microwave Synthesis Introduction In the electromagnetic spectrum the







- Slides: 25

Microwave Synthesis

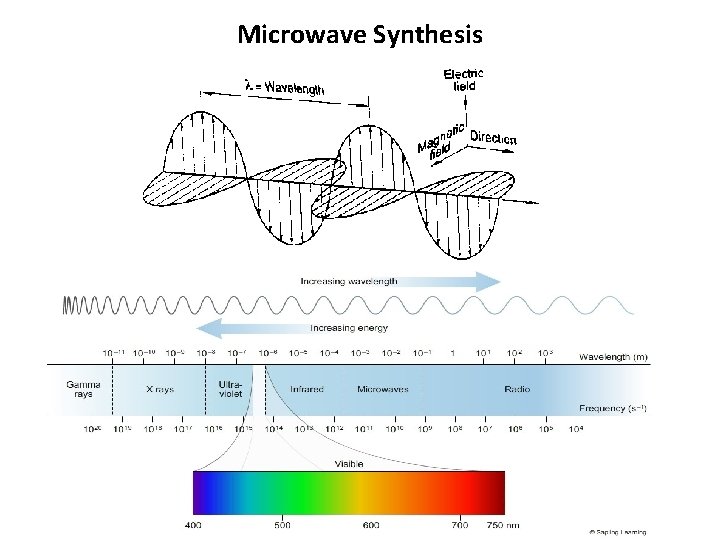
Introduction • In the electromagnetic spectrum the microwave radiation region is located between infrared radiation and radio-waves. • Telecommunication and microwave radar equipment occupy many of the band frequencies in this region. In order to avoid interference with these systems, • the household and industrial microwave ovens operate at a fixed frequency of 2. 45 GHz. [17– 19] • The energy of the quantum involved can be calculated by the Planck’s law E = h ν and is found to be 0. 3 cal mol– 1.

Conventional heating • In this method of heating, reactants are slowly activated by a conventional external heat source. • Heat is driven into the substance, passing first through the walls of the vessel in order to reach the solvent and the reactants. • This is a slow and inefficient method for transferring energy into the reacting system.

Microwave heating • Here, microwaves couple directly with the molecules of the entire reaction mixture, leading to a rapid rise in the temperature. • As process is not dependant on thermal conductivity of the vessel, the result is an instantaneous localized superheating of any substance • that will respond to either dipole rotation or ionic conductivity. • Only the reaction vessel contents are heated and not the vessel itself • Results better homogeneity and selective heating of polar molecules

Principles of Microwave Activation • The acceleration of chemical reactions by microwave exposure results from the interactions between the material and electromagnetic field leading to thermal and specific (non-thermal) effects. (1) Dipole interactions • For microwave heating, the substance must possess a dipole moment. • A dipole is sensitive to external electric field and tries to align itself with the field by rotation. • If submitted to an alternating current, the electric field is inversed at each alterance and • therefore dipoles tend to move together to follow the inversed electric field. • Such a characteristic induces rotation and friction of the molecules, which dissipates as internal homogeneous heating.

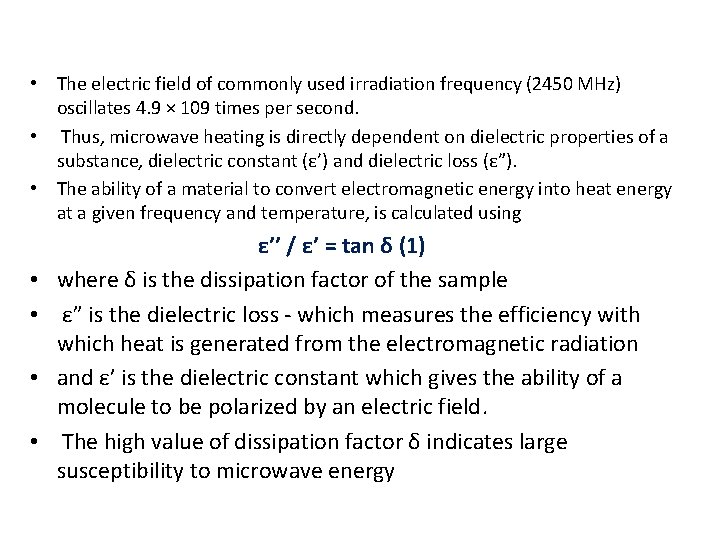
• The electric field of commonly used irradiation frequency (2450 MHz) oscillates 4. 9 × 109 times per second. • Thus, microwave heating is directly dependent on dielectric properties of a substance, dielectric constant (ε’) and dielectric loss (ε”). • The ability of a material to convert electromagnetic energy into heat energy at a given frequency and temperature, is calculated using • • ε’’ / ε’ = tan δ (1) where δ is the dissipation factor of the sample ε” is the dielectric loss - which measures the efficiency with which heat is generated from the electromagnetic radiation and ε’ is the dielectric constant which gives the ability of a molecule to be polarized by an electric field. The high value of dissipation factor δ indicates large susceptibility to microwave energy

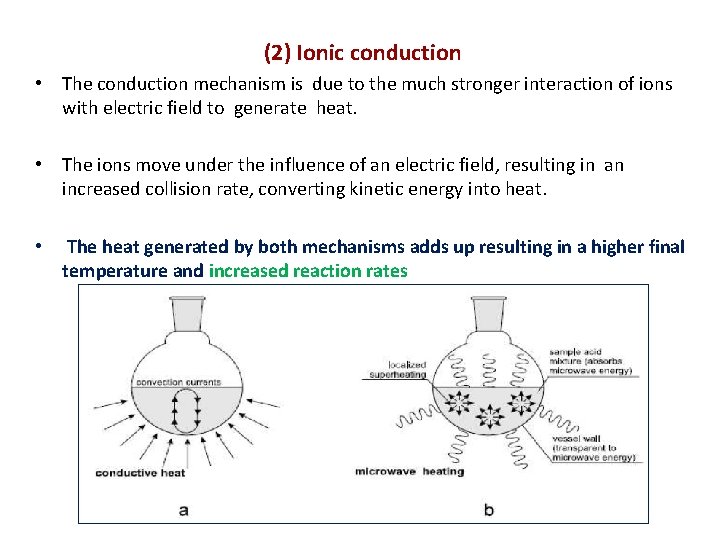
(2) Ionic conduction • The conduction mechanism is due to the much stronger interaction of ions with electric field to generate heat. • The ions move under the influence of an electric field, resulting in an increased collision rate, converting kinetic energy into heat. • The heat generated by both mechanisms adds up resulting in a higher final temperature and increased reaction rates

MICROWAVE INDUCED SUPERHEATED BOILING OF SOLVENTS • • • Conventional heating In organic synthesis, it is common practise to carry out reactions under reflux conditions. The boiling ensures a good mixing and the highest possible temperature for the solvent at atmospheric pressure. The temperature of a boiling solvent is normally assumed to be exactly at the point were the partial vapour pressure of the solvent is equal to 1 bar. However, this is not necessarily the case. During reflux, solvent continuously evaporates, condenses and flows back into the reaction pool. Hence, the system is in a steady state rather than in equilibrium and the temperature is not exactly at the equilibrium boiling point. MICROWAVE ASSISTED HETEROGENEOUS AND HOMOGENEOUS REACTIONS Farid CHEMAT (1) and Erik ESVELD (2) Fifth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-5), http: //www. mdpi. org/ecsoc-5. htm, 1 -30 September 2001

SUPERHEATING or SUPERBOILING • In a microwave heating, the average temperature of the solvent can be at higher temperature than the atmospheric boiling point. • This is because microwave power is dissipated over the whole volume of the solvent, where nucleation points neccessary for boiling are absent. • The loss of the excess of thermal energy by boiling can therefore only occur at the side of the reactor or at the solvent-air interface. • • This results in a reversed temperature profile with a steady average reflux temperature above the classical boiling point. • This is called super boiling. • With the same reactor overheating is not observed under conventional heating (Mingos and Baghurst, 1992).

Superheating advantage • The microwave superheating phenomena can be used to accelerate homogeneous chemical reactions • Therefore, reaction times can be reduced from days under classical heating to minutes under microwave heating • In comparison with the classical process, the volume of the reactor, time, waste reject and the amount of solvent required are reduced. • • • Factors affecting microwave heated super boiling the physical properties of the solvent the reactor geometry the mass flow the heat flow and the electric field distribution. Among the solvent properties, the assosiative properties and dielectric properties are of prime importance.

Effects of solvents in microwave assisted synthesis • compounds with high dielectric constants such as water, ethanol, acetonitrile, N, N-dimethylformamide (DMF), acetic acid, chloroform. dichloromethane, acetone, ethylene glycol etc. , tend to heat rapidly under microwave irradiation. • while less polar substances, such as aromatic and aliphatic hydrocarbons or compounds with no net dipole moment, such as carbon dioxide, carbon tetrachloride, diethyl ether etc. are poorly absorbing.

Microwave technology in process optimization

Microwave technology applications in various organic reactions and heterocycles synthesis • Chemists have successfully conducted a large range of organic reactions. These include : 8. Esterification • 1. Diels-Alder reaction 2. Heck reaction 3. Suzuki reaction 4. Mannich reaction 5. Hydrogenation of [beta]-lactams 6. Hydrolysis 7. Dehydration 9. Cycloaddition reaction 10. Epoxidation 11. Reductions 12. Condensations 13. Protection and deprotection 14. Cyclisation reactions. • Microwave-assisted organic synthesis is being widely applied for developing compounds in the lead optimization phase in the pharmaceuticals industry.

Organic synthesis at atmospheric pressure • Microwave-assisted organic reactions can be conveniently conducted at atmospheric pressure in reflux conditions • e. g. Diels-Alder reaction of maleic anhydride with anthracene. In the presence of diglyme (boiling point 162 ºC) • this reaction can be completed in a minute, with a 90% yield • However, the conventional synthetic route, which uses benzene, requires 90 minutes. • High boiling solvents are preferred in microwave assisted organic synthetic reactions.

Organic synthesis at elevated pressure • Microwaves can be used to directly heat the reaction mixture in sealed microwave-transparent containers. • The sealed container helps in increasing the pressure in the reactor, which facilitates the reaction • This results in a substantial increase in the reaction rate • However, increase in the reaction rate of any chemical synthesis depends on three factors • Volume of the vessel, the solvent to space ratio, and the solvent boiling point.

Organic synthesis in dry media • Microwaves have been applied to organic synthesis in dry media, using solid supports • i. e. alumina, montmorillonite clay, alkali metal fluoride doped alumina and silica • Or strongly absorbing (i. e. , graphite) inorganic support • Microwave radiation, based on solid supports, has been highly successful in reducing the reaction time • E. g. condensation, acetylation and deacetylation reactions • deacetylation of a protected compound such as alcoholic acetate held on a support material. • The microwave assisted reaction could be completed within two to three minutes, compared to conventional oil-bath heating at 75 °C for 40 hours

The first reports • Use of microwave heating to accelerate organic chemical transformations (MAOS) were published by the groups of Richard Gedye and Raymond J. Giguere/George Majetich in 1986.

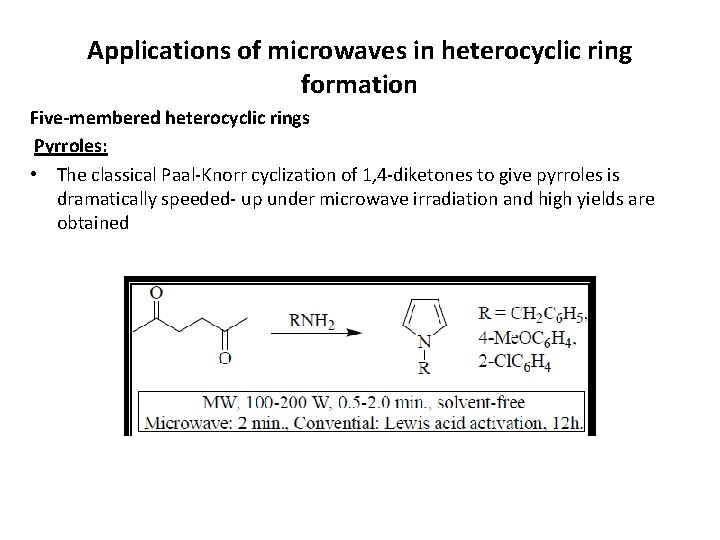
Applications of microwaves in heterocyclic ring formation Five-membered heterocyclic rings Pyrroles: • The classical Paal-Knorr cyclization of 1, 4 -diketones to give pyrroles is dramatically speeded- up under microwave irradiation and high yields are obtained

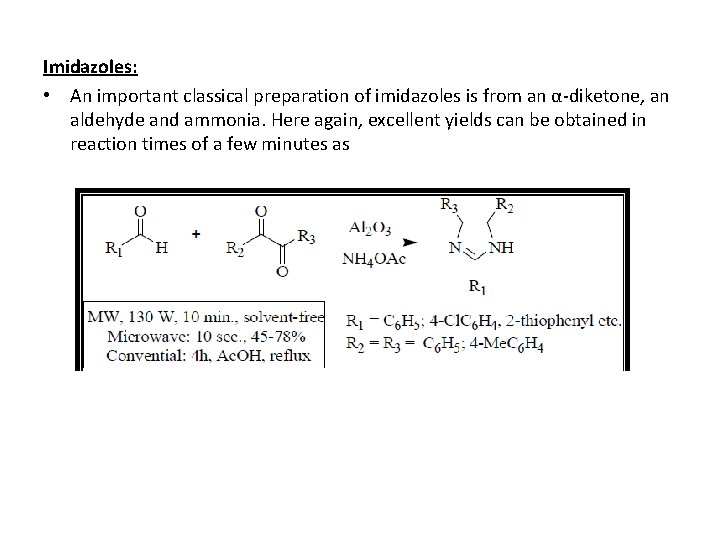
Imidazoles: • An important classical preparation of imidazoles is from an α-diketone, an aldehyde and ammonia. Here again, excellent yields can be obtained in reaction times of a few minutes as

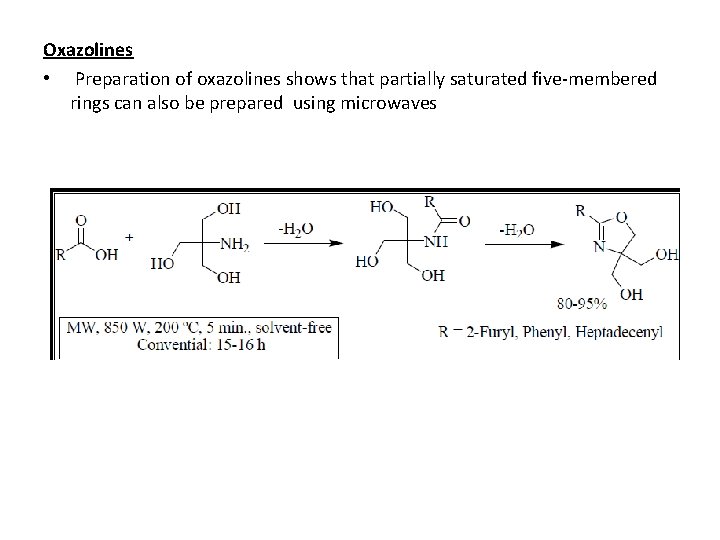
Oxazolines • Preparation of oxazolines shows that partially saturated five-membered rings can also be prepared using microwaves

Tetrazoles

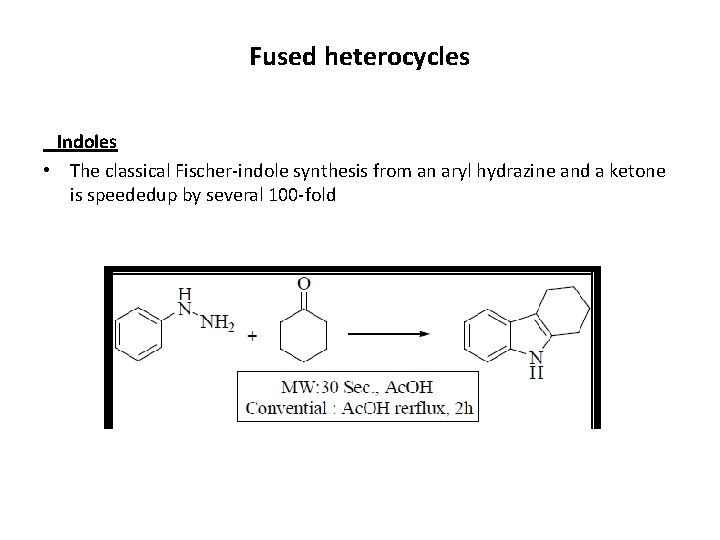
Fused heterocycles Indoles • The classical Fischer-indole synthesis from an aryl hydrazine and a ketone is speededup by several 100 -fold

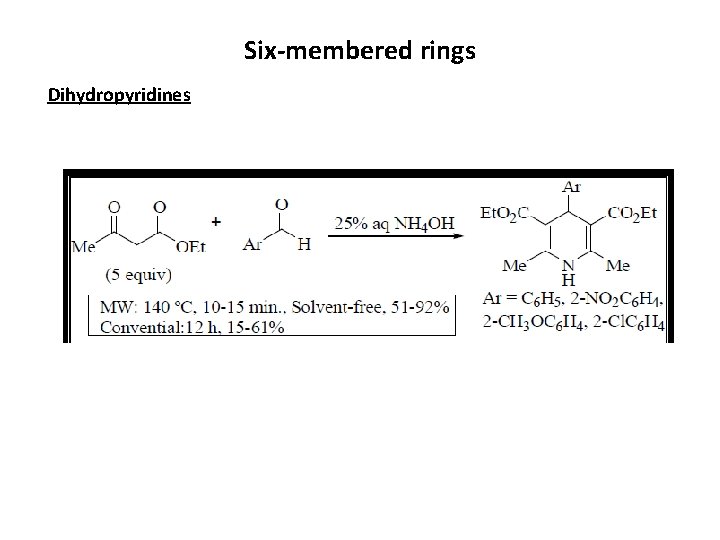
Six-membered rings Dihydropyridines

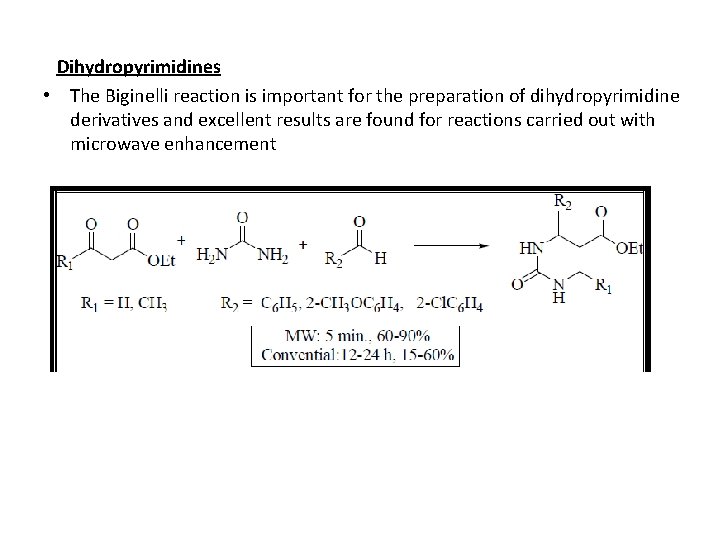
Dihydropyrimidines • The Biginelli reaction is important for the preparation of dihydropyrimidine derivatives and excellent results are found for reactions carried out with microwave enhancement

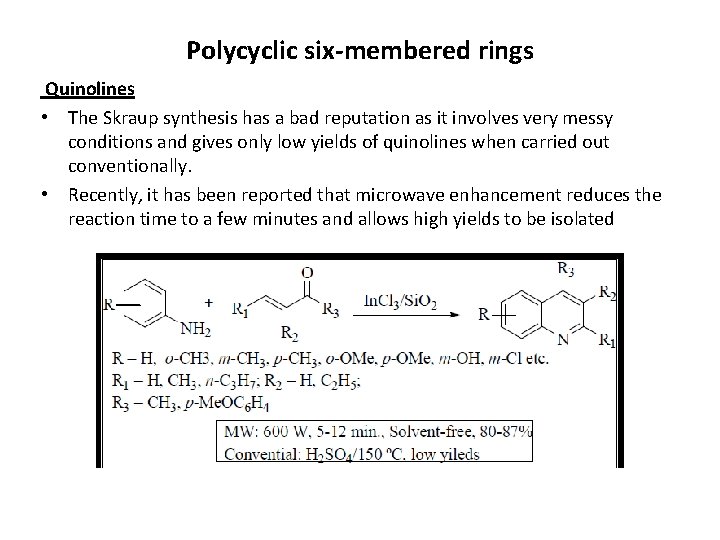
Polycyclic six-membered rings Quinolines • The Skraup synthesis has a bad reputation as it involves very messy conditions and gives only low yields of quinolines when carried out conventionally. • Recently, it has been reported that microwave enhancement reduces the reaction time to a few minutes and allows high yields to be isolated