Microstructure and hydrogen storage properties of Fe Ti






- Slides: 12

Microstructure and hydrogen storage properties of Fe. Ti + x wt. % Hf alloys (x = 0, 4, 8 and 12) By : Volatiana RAZAFINDRAMANANA Jean-Louis BOBET and Jacques HUOT At the 2016 CAP Congress – 15 th of June 2016

Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion 1. State of art • Renewable energy • Hydrogen 2. Metal Hydride • Fe. Ti alloy 3. Fe-Ti-Hf system • Microstructure • First hydrogenation properties 4. Conclusion • Outlook • Perspectives 2

Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion Energy : big challenge of the XXI century High population growth Renewable energy Limited reserves of fossil fuel Reduction in emissions of greenhouse gases Urban transport Electronic HYDROGEN « Stationary » application 3

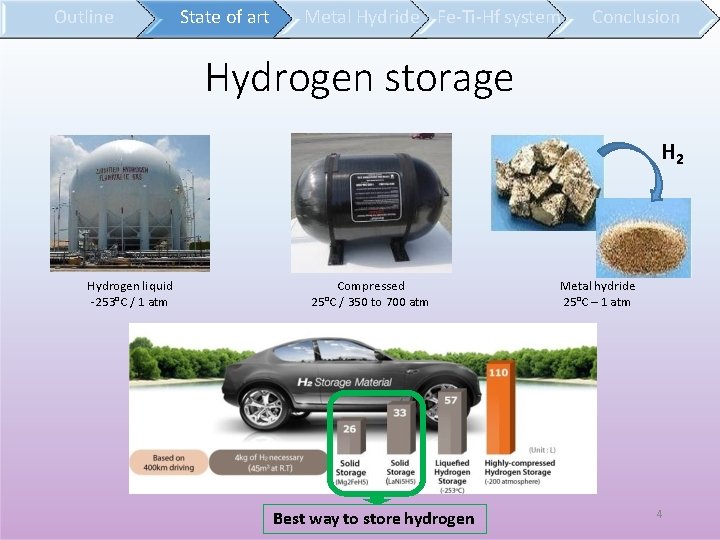
Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion Hydrogen storage H 2 Hydrogen liquid -253°C / 1 atm Compressed 25°C / 350 to 700 atm Best way to store hydrogen Metal hydride 25°C – 1 atm 4

Outline State of art Metal Hydride Fe-Ti-Hf system Metal hydride Metal 1 Conclusion 3 2 1. Adsorption (and dissociation) of hydrogen 2. Diffusion of hydrogen (absorption) 3. Total absorption of hydrogen in the material 5

Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion Fe. Ti alloy Fe Ti Space group Pm 3 m 6

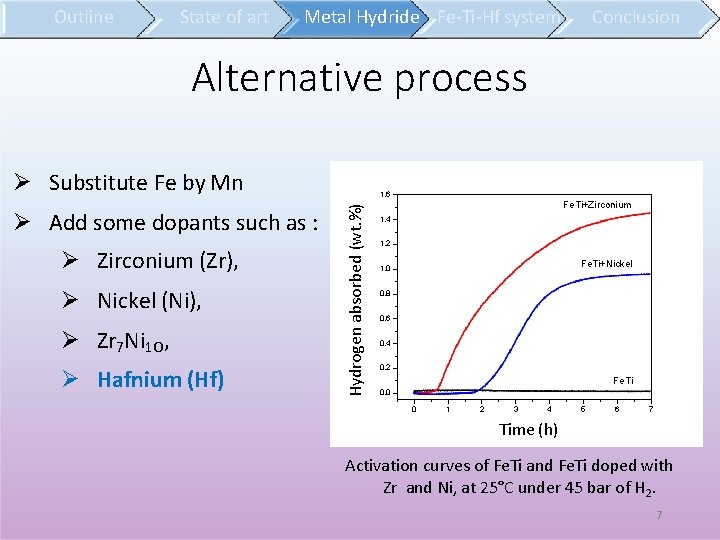
Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion Alternative process Substitute Fe by Mn Zirconium (Zr), Nickel (Ni), Zr 7 Ni 1 O, Hafnium (Hf) Hydrogen absorbed (wt. %) Add some dopants such as : 1, 6 )l a t o ts d i o p (% e n è g ro yd H Fe. Ti+Zirconium 1, 4 1, 2 Fe. Ti+Nickel 1, 0 0, 8 0, 6 0, 4 0, 2 Fe. Ti 0, 0 0 1 2 3 4 5 6 7 Time (h) Temps (h) Activation curves of Fe. Ti and Fe. Ti doped with Zr and Ni, at 25°C under 45 bar of H 2. 7

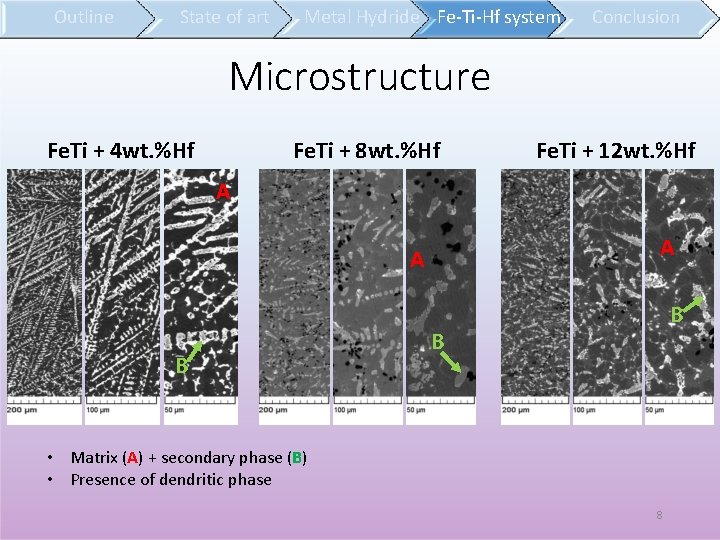
Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion Microstructure Fe. Ti + 8 wt. %Hf Fe. Ti + 4 wt. %Hf Fe. Ti + 12 wt. %Hf A A A B B B • Matrix (A) + secondary phase (B) • Presence of dendritic phase 8

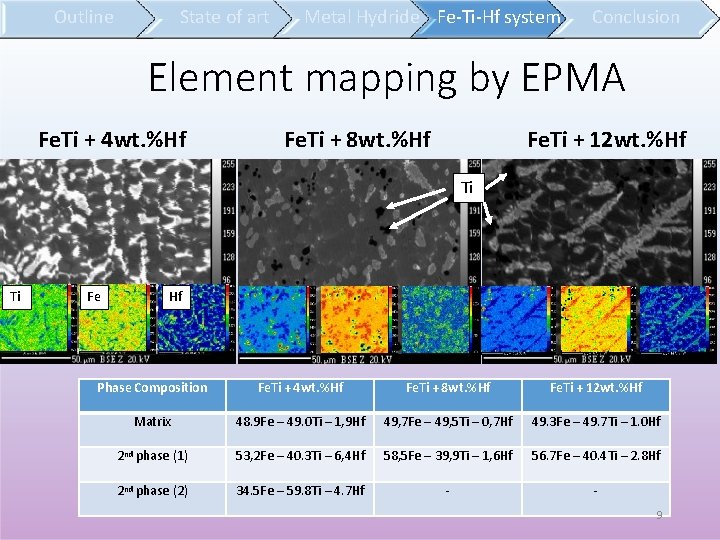
State of art Outline Metal Hydride Fe-Ti-Hf system Conclusion Element mapping by EPMA Fe. Ti + 4 wt. %Hf Fe. Ti + 8 wt. %Hf Fe. Ti + 12 wt. %Hf Ti Ti Fe Hf Phase Composition Fe. Ti + 4 wt. %Hf Fe. Ti + 8 wt. %Hf Fe. Ti + 12 wt. %Hf Matrix 48. 9 Fe – 49. 0 Ti – 1, 9 Hf 49, 7 Fe – 49, 5 Ti – 0, 7 Hf 49. 3 Fe – 49. 7 Ti – 1. 0 Hf 2 nd phase (1) 53, 2 Fe – 40. 3 Ti – 6, 4 Hf 58, 5 Fe – 39, 9 Ti – 1, 6 Hf 56. 7 Fe – 40. 4 Ti – 2. 8 Hf 2 nd phase (2) 34. 5 Fe – 59. 8 Ti – 4. 7 Hf - 9

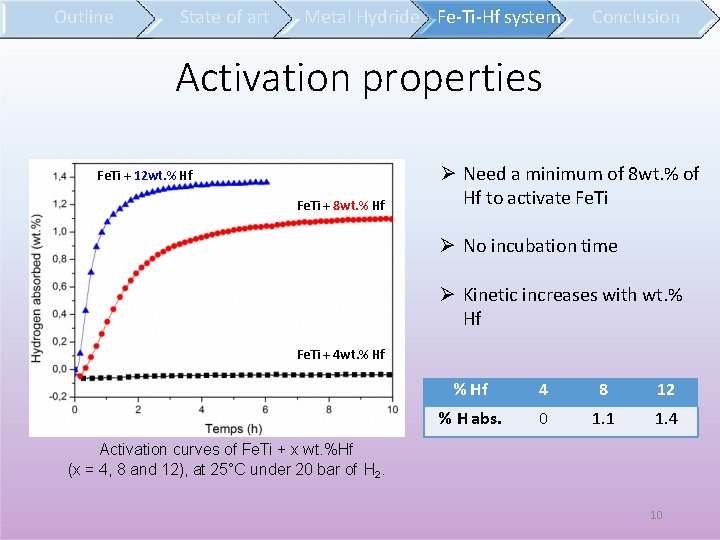
Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion Activation properties Fe. Ti + 12 wt. % Hf Fe. Ti + 8 wt. % Hf Need a minimum of 8 wt. % of Hf to activate Fe. Ti No incubation time Kinetic increases with wt. % Hf Fe. Ti + 4 wt. % Hf 4 8 12 % H abs. 0 1. 1 1. 4 Activation curves of Fe. Ti + x wt. %Hf (x = 4, 8 and 12), at 25°C under 20 bar of H 2. 10

Outline State of art Metal Hydride Fe-Ti-Hf system Conclusion Resume Fe. Ti needs a minimum of 8 wt. % Hf to be activated • Role of the Ti rich phase Fe. Ti alloy doped with hafnium has a good industrial base • Low cost • First hydrogenation at room temperature • Fast kinetic Perspective • Identify the 2 nd phase by neutron diffraction 11

CAP Congress organizers J. Huot & JL. Bobet Thank you for your attention Region Aquitaine My colleagues Questions ?
 Microstructure of a dictionary
Microstructure of a dictionary Lever rule fe-c phase diagram
Lever rule fe-c phase diagram Development of microstructure in isomorphous alloys
Development of microstructure in isomorphous alloys Market microstructure trading strategies
Market microstructure trading strategies Microstructure of ferrous metals
Microstructure of ferrous metals High frequency market microstructure
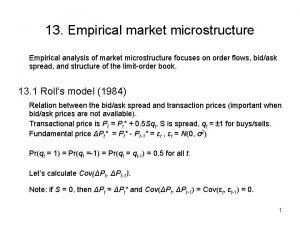
High frequency market microstructure Empirical market microstructure
Empirical market microstructure Hydrogen storage
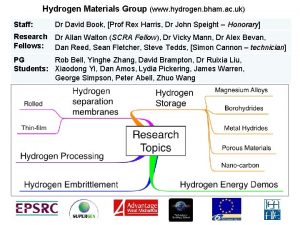
Hydrogen storage Hydrogen storage
Hydrogen storage Hydrogen strategy
Hydrogen strategy Properties of hydrogen
Properties of hydrogen Hydrogen gas test equation
Hydrogen gas test equation Hydrogen bond
Hydrogen bond