Microscopic Compatibility between Methanol and Water in Hydrogen

Microscopic Compatibility between Methanol and Water in Hydrogen Bond Network Development in Protonated Clusters Asuka Fujii, Ken-ichiro Suhara, Kenta Mizuse, Naohiko Mikami Department of Chemistry, Graduate School of Science, Tohoku University, Japan Jer-Lai Kuo School of Physical and Mathematical Sciences, National Nanyang Technological University, Singapore
Hydrogen bond network structures in protonated clusters • microscopic picture of protic solvents • proton motion in liquids • nature of hydrogen bond, …. . , etc. Mass spectrometry size distribution and magic numbers IR dissociation spectroscopy of size-selected cluster cations (Y. T. Lee, 1989) direct probing of the H-bond network structure ab initio and MD calculations Network structure in large-sized clusters
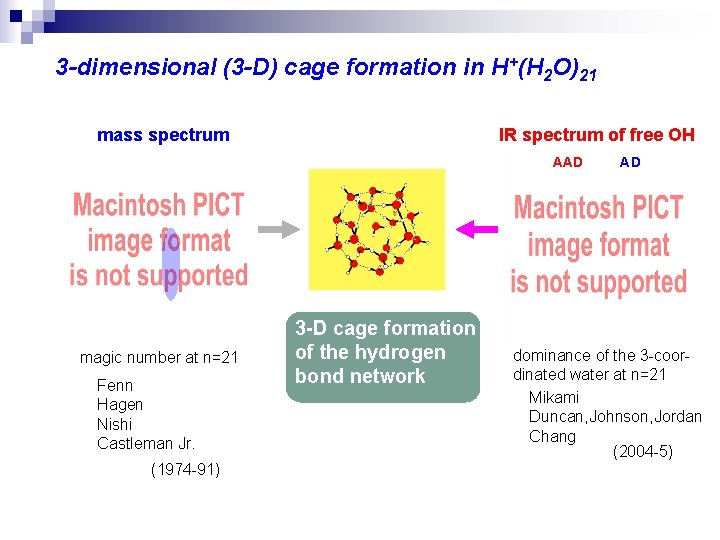
3 -dimensional (3 -D) cage formation in H+(H 2 O)21 mass spectrum IR spectrum of free OH AAD magic number at n=21 Fenn Hagen Nishi Castleman Jr. (1974 -91) 3 -D cage formation of the hydrogen bond network AD dominance of the 3 -coordinated water at n=21 Mikami Duncan, Johnson, Jordan Chang (2004 -5)
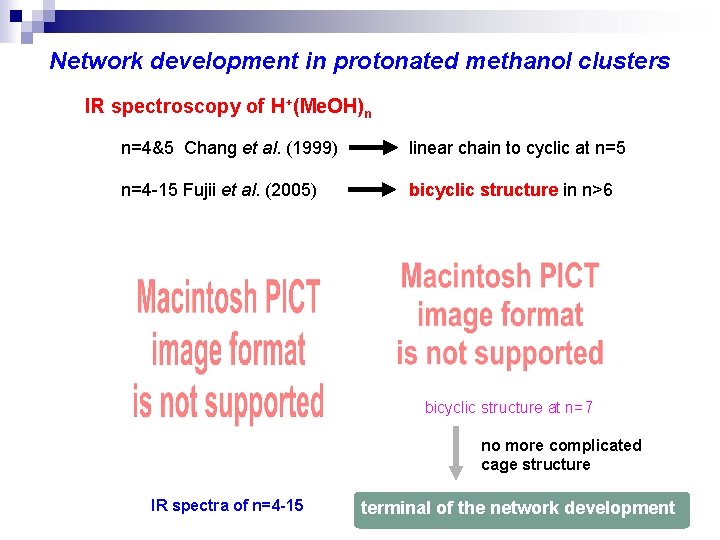
Network development in protonated methanol clusters IR spectroscopy of H+(Me. OH)n n=4&5 Chang et al. (1999) linear chain to cyclic at n=5 n=4 -15 Fujii et al. (2005) bicyclic structure in n>6 bicyclic structure at n=7 no more complicated cage structure IR spectra of n=4 -15 terminal of the network development
Hydrogen bond network in protonated water-methanol mixed clusters water : 4 -coordination, complicated cage methanol : 3 -coordination, simple network hydrogen bond network in the mixed system? What really happens in aqueous alcohol ?
This study IR spectroscopy of H+(Me. OH)m(H 2 O)n m=1 -4, n=4 -22 (m<<n) (@Sendai) (the case of m>>n : FD 05 by K. Suhara) DFT calculations of the relative stabilities of isomer structures (@Singapore) model systems : (Me. OH)m(H 2 O)n (m+n=8) and H+(Me. OH)1(H 2 O)20 Microscopic compatibility between methanol and water in H-bond network formation
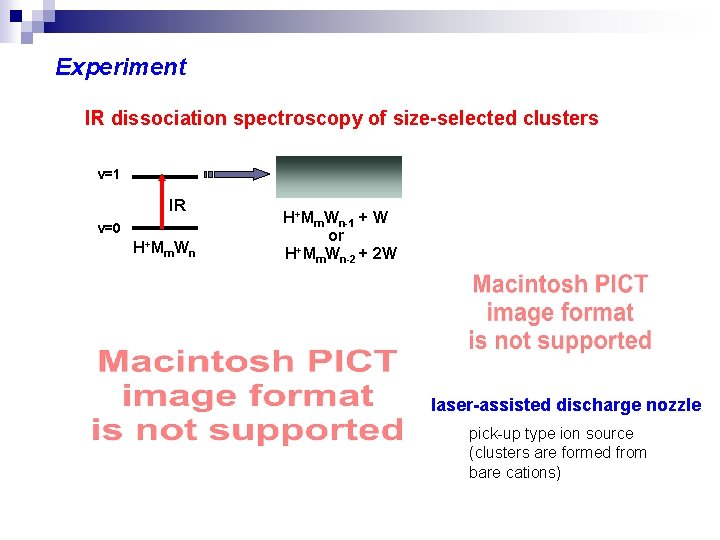
Experiment IR dissociation spectroscopy of size-selected clusters v=1 IR v=0 H+Mm. Wn-1 + W or + H Mm. Wn-2 + 2 W laser-assisted discharge nozzle pick-up type ion source (clusters are formed from bare cations)
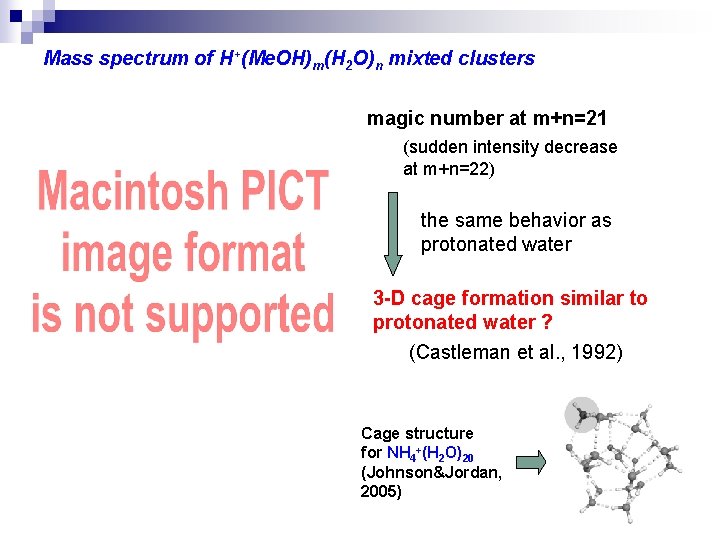
Mass spectrum of H+(Me. OH)m(H 2 O)n mixted clusters magic number at m+n=21 (sudden intensity decrease at m+n=22) the same behavior as protonated water 3 -D cage formation similar to protonated water ? (Castleman et al. , 1992) Cage structure for NH 4+(H 2 O)20 (Johnson&Jordan, 2005)
Infrared spectra of H+(Me. OH)2(H 2 O)n OH stretching vibrational region H-bonded OH stretch : broadened free OH stretch : more informative
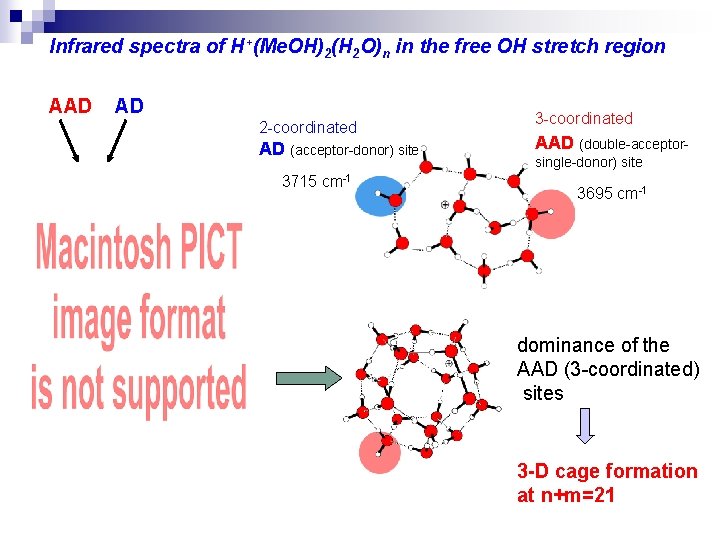
Infrared spectra of H+(Me. OH)2(H 2 O)n in the free OH stretch region AAD AD 2 -coordinated AD (acceptor-donor) site 3715 cm-1 3 -coordinated AAD (double-acceptorsingle-donor) site 3695 cm-1 dominance of the AAD (3 -coordinated) sites 3 -D cage formation at n+m=21
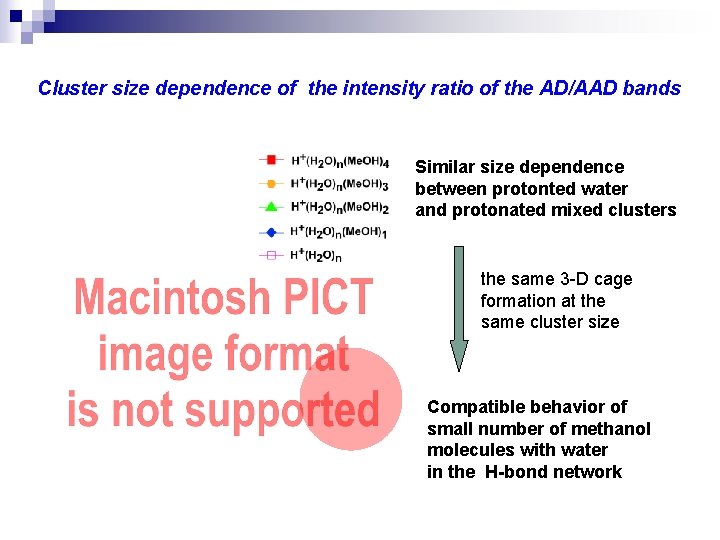
Cluster size dependence of the intensity ratio of the AD/AAD bands Similar size dependence between protonted water and protonated mixed clusters the same 3 -D cage formation at the same cluster size Compatible behavior of small number of methanol molecules with water in the H-bond network
Theoretical confirmation of the microscopic compatibility model system : neutral (H 2 O)8 smallest polyhedral cage (cube) 14 orientational isomers substitution of all the AAD sites with methanol (Me. OH)4(H 2 O)4 DFT evaluation of the relative stabilization energies (B 3 LYP/6 -31+G*)
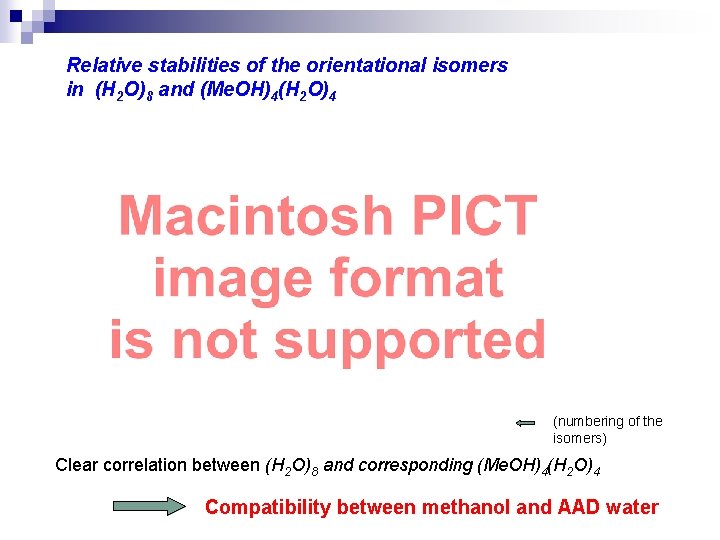
Relative stabilities of the orientational isomers in (H 2 O)8 and (Me. OH)4(H 2 O)4 (numbering of the isomers) Clear correlation between (H 2 O)8 and corresponding (Me. OH)4(H 2 O)4 Compatibility between methanol and AAD water
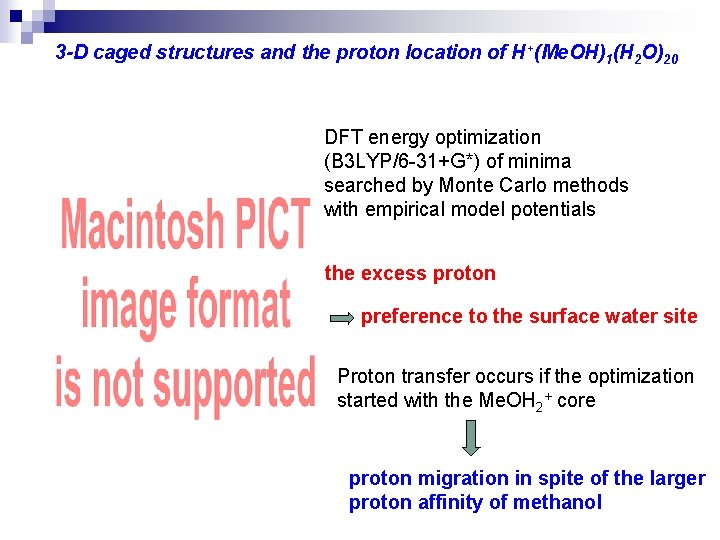
3 -D caged structures and the proton location of H+(Me. OH)1(H 2 O)20 DFT energy optimization (B 3 LYP/6 -31+G*) of minima searched by Monte Carlo methods with empirical model potentials the excess proton preference to the surface water site Proton transfer occurs if the optimization started with the Me. OH 2+ core proton migration in spite of the larger proton affinity of methanol
Summary IR spectroscopy of H+(Me. OH)m(H 2 O)n (m=1 -4, n=4 -22) in the OH stretch region Spectroscopic evidence for the 3 -D cage formation of the mixed clusters at the magic number m+n=21 (m=1 -4) Microscopic compatibility between methanol and AAD water in the H-bond network development DFT calculations also support the compatibility in the relative energy The excess proton prefers the surface water site in the (1+20)-mer indicating the proton migration from methanol to water
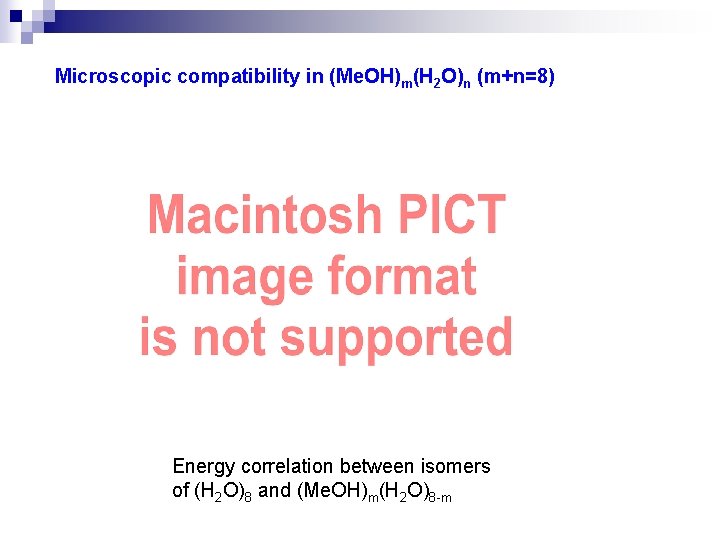
Microscopic compatibility in (Me. OH)m(H 2 O)n (m+n=8) Energy correlation between isomers of (H 2 O)8 and (Me. OH)m(H 2 O)8 -m
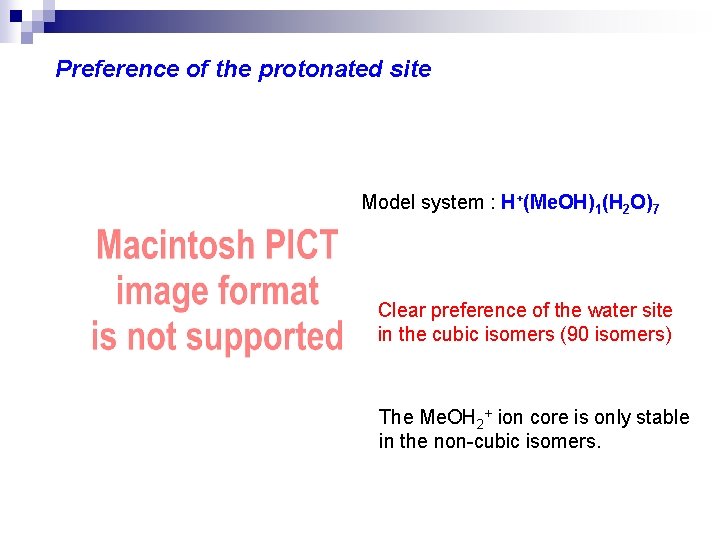
Preference of the protonated site Model system : H+(Me. OH)1(H 2 O)7 Clear preference of the water site in the cubic isomers (90 isomers) The Me. OH 2+ ion core is only stable in the non-cubic isomers.

- Slides: 18