Micropatterning Thin Polystyrene Films for Single Cell Culture










- Slides: 18

Micropatterning Thin Polystyrene Films for Single Cell Culture Biological Microsystems Lab Dr. David Eddington Elly Sinkala Krina Gandhi

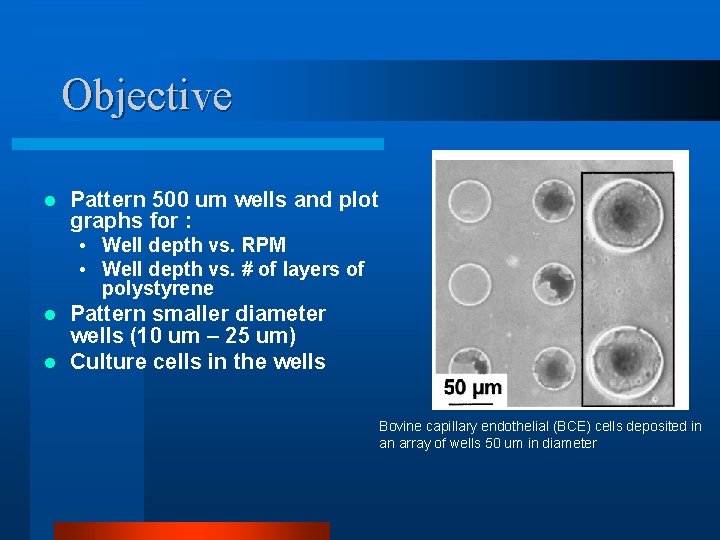
Objective l Pattern 500 um wells and plot graphs for : • Well depth vs. RPM • Well depth vs. # of layers of polystyrene Pattern smaller diameter wells (10 um – 25 um) l Culture cells in the wells l Bovine capillary endothelial (BCE) cells deposited in an array of wells 50 um in diameter

Motivation Show that we can culture a single cell in one well l Control cell-to-cell interactions l Control cell shape l Applications: l • Study cell characteristics • Drug testing • Tissue engineering

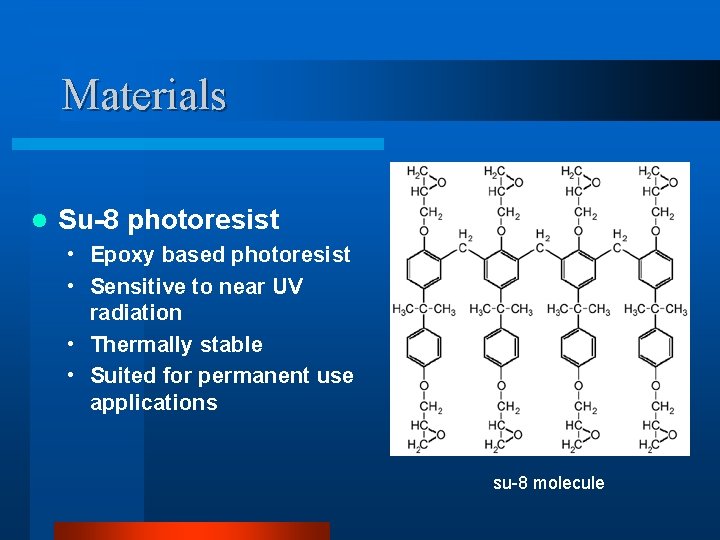
Materials l Su-8 photoresist • Epoxy based photoresist • Sensitive to near UV radiation • Thermally stable • Suited for permanent use applications su-8 molecule

Materials l Polydimethylsiloxane (PDMS) • • Non-toxic Hydrophobic Transparent Readily available Curing agent Elastomer base

Materials l Polystyrene • Clear, colorless polymer • Easily available • Cost effective

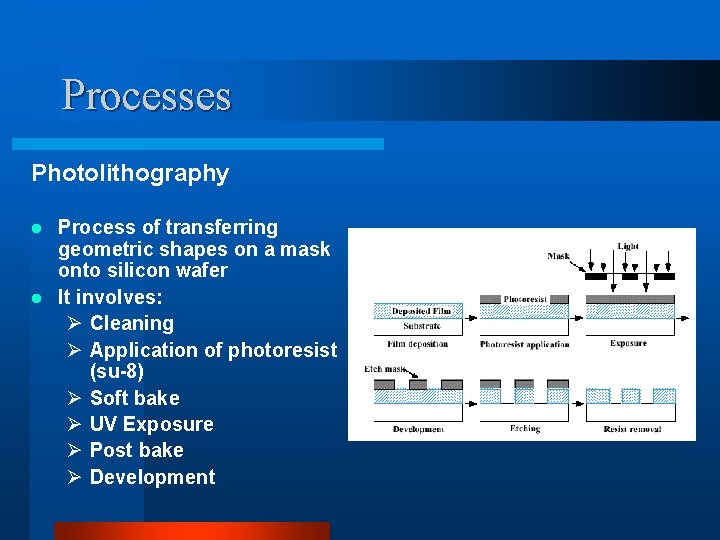
Processes Photolithography Process of transferring geometric shapes on a mask onto silicon wafer l It involves: Ø Cleaning Ø Application of photoresist (su-8) Ø Soft bake Ø UV Exposure Ø Post bake Ø Development l

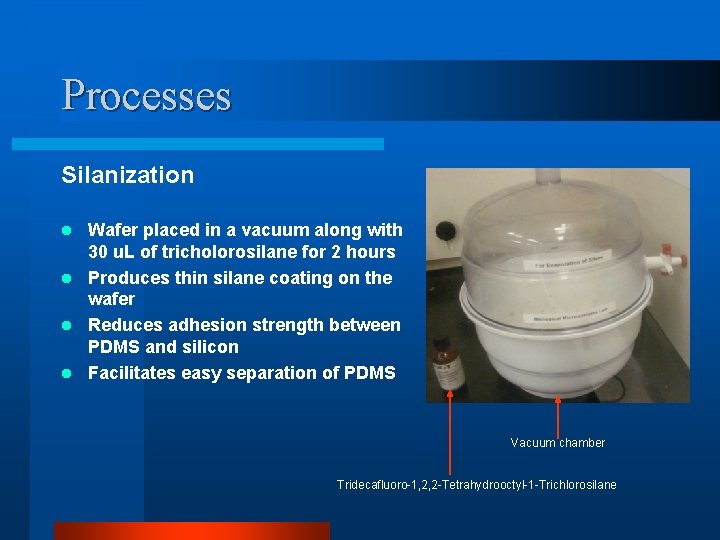
Processes Silanization Wafer placed in a vacuum along with 30 u. L of tricholorosilane for 2 hours l Produces thin silane coating on the wafer l Reduces adhesion strength between PDMS and silicon l Facilitates easy separation of PDMS l Vacuum chamber Tridecafluoro-1, 2, 2 -Tetrahydrooctyl-1 -Trichlorosilane

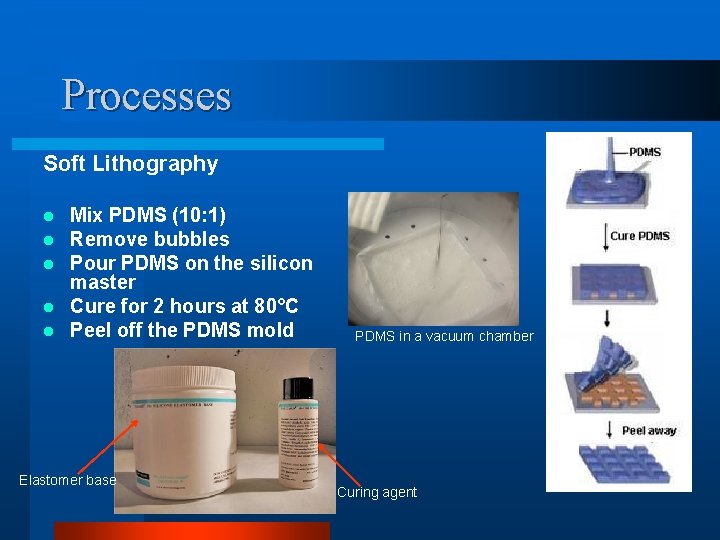
Processes Soft Lithography Mix PDMS (10: 1) Remove bubbles Pour PDMS on the silicon master l Cure for 2 hours at 80°C l Peel off the PDMS mold l l l Elastomer base PDMS in a vacuum chamber Curing agent

Processes Embossing Glass cover slips coated with polystyrene Spin polystyrene on glass cover slip l Place PDMS mold over it l Place it on a hot plate for 10 min at 180°C l Peel it off once cooled l PDMS mold

Processes Well Depth Measurement Height vs. Diameter plot generated using data from profilometer Tencore Profilometer

Processes Cell Culture • Sterilized the wells • Seeded cells (MDCK-Madin-Darby Canine Kidney Cells) • Allowed cells to adhere (3 -4 hours) • Washed excess cells with PBS • Filled the wells with media

Results

Results

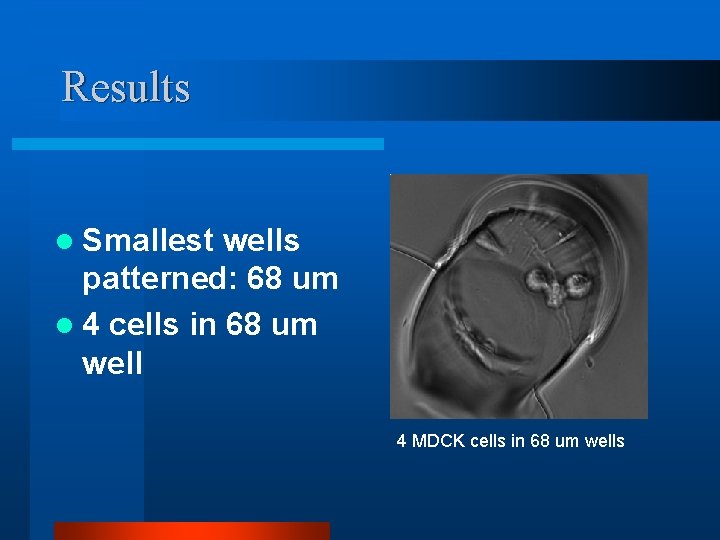
Results l Smallest wells patterned: 68 um l 4 cells in 68 um well 4 MDCK cells in 68 um wells

Conclusion l Collected data and plotted them for: • Well depth vs. RPM • Well depth vs. # of layers Smallest wells patterned: 68 um l Was able to culture cells l Future Work Continue to work on smaller diameter wells l Try to culture one cell in one well using the smaller wells l

Acknowledgments l l l Dr. David Eddington Elly Sinkala Dr. Takoudis Dr. Jursich National Science Foundation (NSF) Department of Defense (DOD) EEC-NSF Grant # 07551150

References l l l "Single Cell Localization and Patterning. " Rapid Prototyping Laboratory for energy and biology. 2007. Stanford University. 29 July 2009 <www-rpl. stanford. edu/. . . /celllocalization/>. Wang, Chun, Fung Ling Yap, and Yong Zhang. "Micropatterning of polystyrene nanoparticles and its bioapplications. " Colloids and Surfaces B: Biointerfaces 46 (2005): 255 -60. Nakanishi, Jun, Tohru Takarada, Kazuo Kamaguchi, and Mizuo Maeda. "Recent Advances in Cell Micropatterning Techniques for. " The Japan Society for Analytical Chemistry 24 (2008): 67 -72. Whitesides, George M. , Emanuele Ostuni, Shuichi Takayama, Xingyu Jiang, and Donald E. Ingber. "Soft lithography in biology and biochemistry. " Annu. Rev. Biomed. Engineering 3 (2001): 335 -73 Yang, Gloria Y. , Vasudev J. Bailey, Yu-Hsin Wen, Gisela Lin, William C. Tang, and Joyce H. Keyak. "Fabrication and Characterization of Microscale Sensors for. " IEEE Xplore (2004): 1355 -358. Questions? ?