Microbiological Culture Media Isolation of bacteria is accomplished

Microbiological Culture Media Isolation of bacteria is accomplished by growing ("culturing") them on the surface of solid nutrient media. Such a medium normally consists of a mixture of protein digests (e. g. , peptone, tryptone) , sugar and inorganic salts, hardened by the addition of 1. 5% agar. Culture media : are solutions containing all the nutrients and necessary physical growth parameters necessary for microbial growth, that are used for culturing of microorganisms (singular, medium). (culture is the microorganisms that grow in a culture medium )

There are 3 physical forms of culture media : 1. Liquid, or broth media (nutrient broth, tryptic soy broth) : - can be used to propagate large numbers of microorganisms, in fermentation studies and for various biochemical tests. 2. Semisolid media(motility test media ): -can also be used in fermentation studies, in determining bacterial motility, and in promoting anaerobic growth.

3. Solid media : -such as nutrient agar or blood agar, are used for (1) surface growth of microorganisms in order to observe colony appearance. (2) pure culture isolations. (3) storage of cultures. (4) to observe specific biochemical reactions. The major difference among these media is that solid and semisolid media contain a solidifying agent (usually agar), whereas a liquid medium does not.
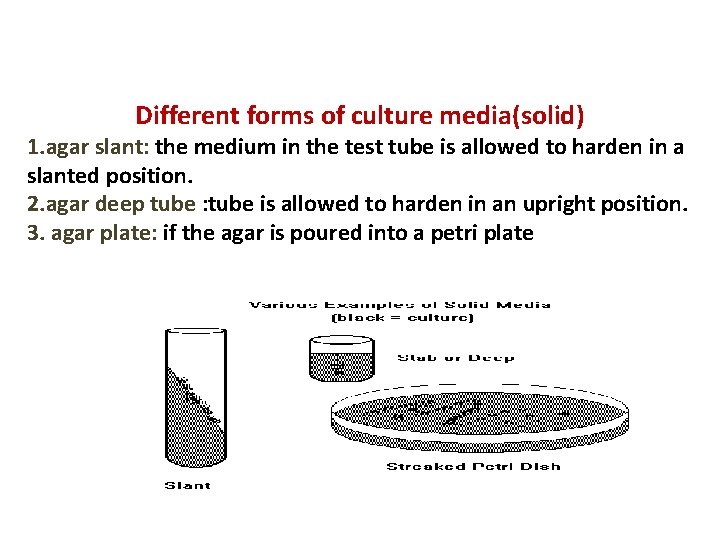
Different forms of culture media(solid) 1. agar slant: the medium in the test tube is allowed to harden in a slanted position. 2. agar deep tube : tube is allowed to harden in an upright position. 3. agar plate: if the agar is poured into a petri plate
![Types of culture media(chemically) 1. Non-synthetic [chemically undefined] medium: contains the nutrient requirements of Types of culture media(chemically) 1. Non-synthetic [chemically undefined] medium: contains the nutrient requirements of](http://slidetodoc.com/presentation_image_h2/1fe1438ef8d35d9b9b9ab222ca94ffc3/image-5.jpg)
Types of culture media(chemically) 1. Non-synthetic [chemically undefined] medium: contains the nutrient requirements of organisms(that are rich in vitamins and nutrients). Nutrient broth, for example, is derived from cultures of yeasts. 2. Chemically defined [synthetic] medium : chemically defined medium is prepared from purified ingredients and therefore whose exact composition is known.
3. According to the function: A. Enriched media: contain the nutrients required to support the growth of a wide variety of organisms, including some of the more fastidious ones. Like blood agar and chocolate agar B. Selective media: permit the growth of one type and prevent the growth of another type of microorganisms because they contain inhibitors such as crystal violet and antibiotics. eg. Mac. Conkey agar. C. Differential media: contain reagents that make differentiation after microorganisms' growth. eg. Eosin methylene blue agar (EMB) for Enterobacteriaceae.

Preparation of culture media Re-hydrate powder according to manufacturer’s instructions. Before sterilization, ensure ingredients are completely dissolved, using heat if necessary. Normally allow 15 -20 cm 3 medium/ Petri dish. Agar slopes are prepared in test tubes or Universal/Mc. Cartney bottles by allowing sterile molten cooled medium to solidify in a sloped position.

Pouring a plate 1. take bottle of sterile molten agar. 2. Hold the bottle in the left hand; remove the lid with the little finger of the right hand. 3. Flame the neck of the bottle. 4. Lift the lid of the Petri dish slightly with the right hand pour the medium into the Petri dish. 5. Flame the neck of the bottle and replace the lid. 6. Allow the plate to solidify. The base of the plate must be covered, agar must not touch the lid of the plate and the surface must be smooth with no bubbles.
Methods of microorganisms' cultivation A. STREAK PLATE METHOD The most common way of separating bacterial cells on the agar surface to obtain isolated colonies is the streak plate method. It provides a simple and rapid method of diluting the sample by mechanical means. As the loop is streaked across the agar surface, more and more bacteria are rubbed off until individual separated organisms are deposited on the agar. After incubation, the area at the beginning of the streak pattern will show confluent growth, while the area near the end of the pattern should show discrete colonies.

PROCEDURE Step 1. Begin with inoculating the first, or primary, quadrant of the agar plate. Don't penetrate or scrape the agar surface. Cover plate with lid. Step 2. Flame the loop, cool by touching an uninoculated portion of the surface. Step 3. Now rotate the plate. Open lid and streak again, following the diagram Remember: you are picking up growth from quadrant one, and using this as your inoculum for quadrant two. Step 4. Flame loop; rotate plate, and repeat procedure for quadrants three and four. The proper wrist action and light touch takes practice. Step 5. Incubate plate inverted (At 37°C for 24 hours)

B. THE POUR PLATE AND SPREAD PLATE METHODS OF ISOLATION This method involves diluting the bacterial sample in tubes of sterile water, saline, or broth 1. Serial Dilution of samples 1)Take 6 dilution tubes, each containing 9 ml of sterile saline. 2) Dilute 1 ml or gram of a sample and dispense this sample into the first tube. 3) withdraw 1 ml from the first dilution tube and dispense into the second dilution tube. Subsequently withdraw 1 ml from the second dilution tube and dispense into the third dilution tube. Continue doing this from tube to tube until the dilution is completed.

2. Plating the diluted samples: there are two main methods of plating : 1 - spread plate method consists of evenly spreading the diluted sample over an agar plate. When using this method, a volume of 0. 1 ml of the diluted sample is transfer to agar surface since the agar will not be able to absorb the excess. Using this method yields colonies that form on the surface of the agar.

2 - Pour plate method, a diluted sample is pipetted into a sterile Petri plate, then melted agar is poured in and mixed with the sample. Using this method allows for a larger volume of the diluted sample(1 ml). This method yields colonies that form colonies throughout the agar, not just on the surface.

2. Incubation of inoculated medium: for bacteria usually 24 -48 hours. 1) Temperature consideration: a. Psychrophiles: under 35°C. b. Mesophiles: 35°C. c. Thermophiles: above 35°C. 2) Atmospheric consideration: a. Aerobes: i. e. air should present during incubation. b. Anaerobes: i. e. air mustn't present. c. Facultative anaerobes: i. e. air present or absent.
Detection of microbial population from rhizosphere Rhizosphere is the extended zone of soil from the root surface to about one centimetre or more (i. e. rhizosphere = the soil at the root surface). Generally soil microorganisms are important components of terrestrial ecosystems where they serve as both recyclers of organic materials and a source of food for organisms.

sample collection A sample of soil can be collected by inserting a sterile spatula into the ground and placing the soil obtained into a sterile petri plate for transport to the laboratory. Note: After the sample has been collected, apply serial dilution and incubate the cultivated microorganisms, and then count the microbial population according to the following:

microbial enumeration (population count) 1. Rossi and Cholodny method: this method involves the insertion of a clean slide into the soil, after a suitable incubation period remove the slide and stain it then exam under light microscope. Procedure: 1. label two clean microscope slides, designating the soil and treatment for that slide. There will be two slides for each cup. Insert each slide vertically into its respective cup, leaving 2 cm of each slide projecting above the soil surface (see Figures 3 -4). Do not force the slides as they will break. 2. Cover the cups with plastic wrap, securing with a rubber band. Puncture the wrap or foil several times with a probe to allow air in and yet preclude excessive evaporation of moisture

3. Remove the two slides from each cup after seven days by pressing each slide to an inclined position and withdrawing in a manner such that the upper face of the slide is not disturbed. Mark and identify the side to be stained. 4. Gently tap the slide on the bench top to remove large soil particles from the slide surface. Clean the lower face with a damp paper towel and dry the slide at room temperature. 5. Wearing protective goggles, immerse the slide in 40% (v/v) acetic acid for 1– 3 min under a fume hood, holding the slide with forceps. 6. Wash off the excess acid under a gentle stream of water, and cover the surface with phenolic Rose Bengal from a dropper bottle, supporting the slide on a staining rack over a container to catch the excess stain.

7. Stain for 5– 10 minutes, but do not permit the slide to become dry. Add more stain as needed. 8. Gently wash the slide to remove excess stain. Dry and examine the slide microscopically using the oil immersion objective.

Most probable number (MPN): first of all a soil solution is prepared by mixing 1 g of soil with 9 ml sterile distilled water and shaking for a minimum of 10 minutes, then apply tenfold serial dilution until , and then apply 9 tubes MPN procedure. The number of microorganisms is then counted as follow: No. of m. o/1 gm soil = MPN value × dilution factor
- Slides: 20