Microbial Metabolism Biochemical diversity Metabolism Relationships Metabolism Pathways
Microbial Metabolism Biochemical diversity
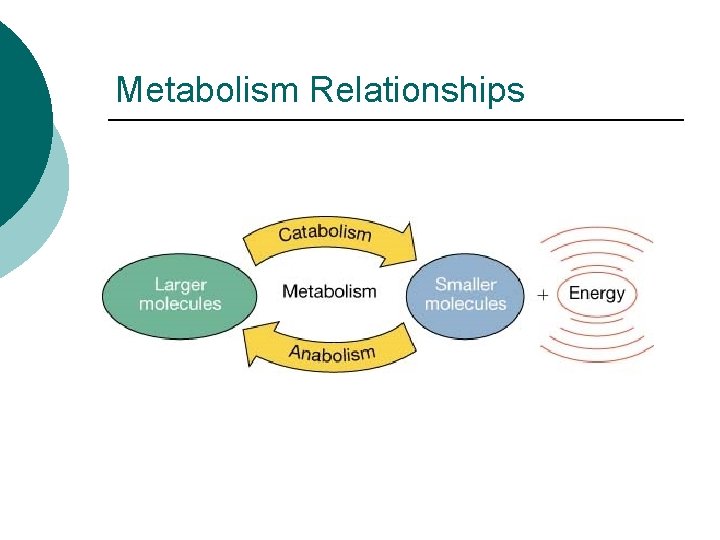
Metabolism Relationships
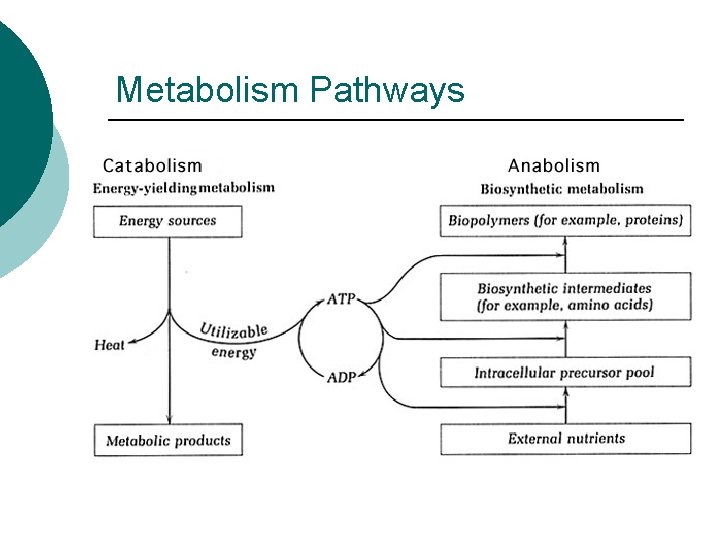
Metabolism Pathways
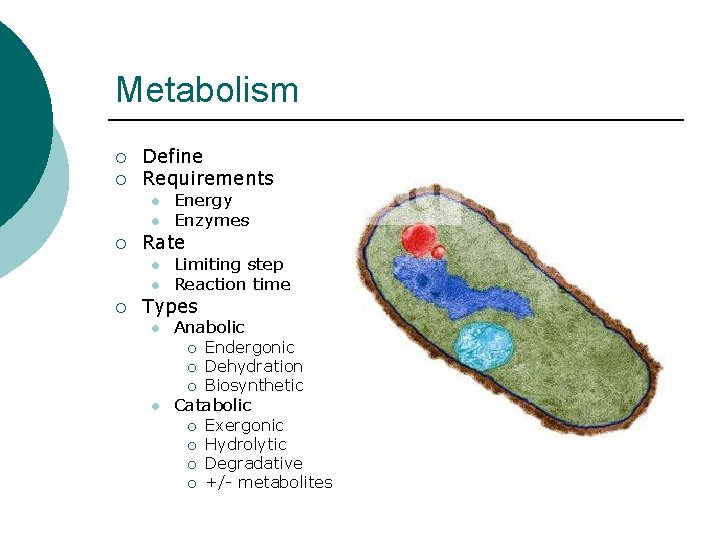
Metabolism ¡ ¡ Define Requirements l l ¡ Rate l l ¡ Energy Enzymes Limiting step Reaction time Types l Anabolic ¡ ¡ ¡ l Endergonic Dehydration Biosynthetic Catabolic ¡ ¡ Exergonic Hydrolytic Degradative +/- metabolites
Metabolic Diversity ¡ Energy generating metabolism l Fermentation ¡ Alcohol ¡ Acid Formation l l ¡ Lactic Acid Mixed Acids Others Respiration ¡ Aerobic ¡ Anaerobic Biosynthesis of secondary metabolites l l Heterotrophic Autotrophic
Energy ¡ Forms l l ¡ Use l l ¡ Chemical Mechanical Electrical Radiation [EM] Chemical Types l l l ¡ Kinetic Potential ATP UTP GTP Heat l l Byproduct 45%
Various Types of Prokaryotic Energy Production Processes Fermentation ¡ Anaerobic Respiration ¡ Aerobic Respiration ¡ Lithotrophy ¡ Photoheterotrophy ¡ Anoxygenic photosynthesis ¡ Methanogenesis ¡
![Enzymes ¡ Structure l l ¡ Protein Ribozyme [ribosome] Characteristic functions l l l Enzymes ¡ Structure l l ¡ Protein Ribozyme [ribosome] Characteristic functions l l l](http://slidetodoc.com/presentation_image/386d3e04144fa713d77e08b85e26ac98/image-8.jpg)
Enzymes ¡ Structure l l ¡ Protein Ribozyme [ribosome] Characteristic functions l l l Active site Specific Modified Forms ¡ ¡ l l Inactive Active Coenzyme/Cofactor -ase Polymerase Others: Lyases, Hydrolases, Isomerases, Transferases
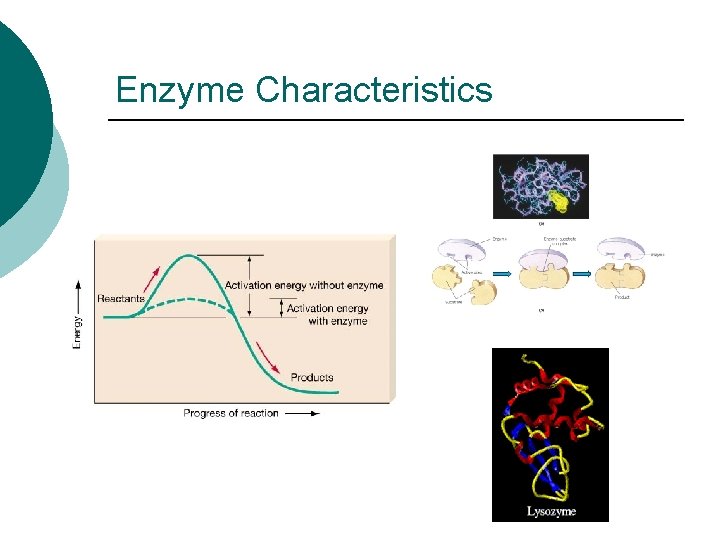
Enzyme Characteristics
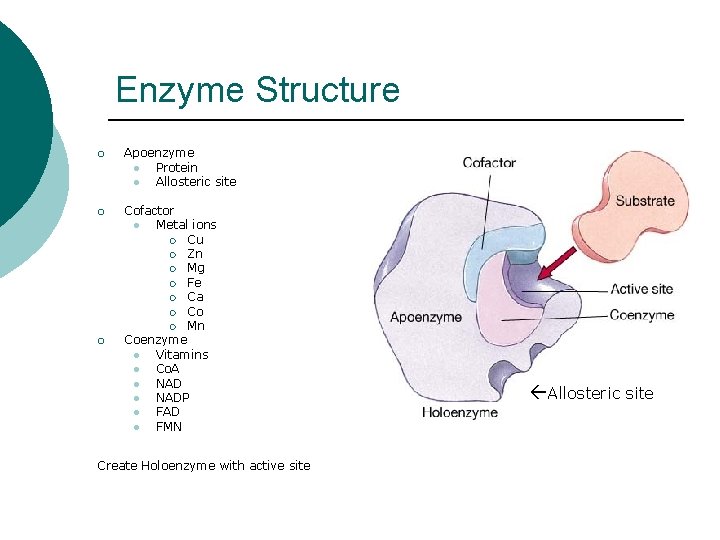
Enzyme Structure ¡ Apoenzyme l Protein l Allosteric site ¡ Cofactor l Metal ions ¡ Cu ¡ Zn ¡ Mg ¡ Fe ¡ Ca ¡ Co ¡ Mn Coenzyme l Vitamins l Co. A l NADP l FAD l FMN ¡ Create Holoenzyme with active site Allosteric site
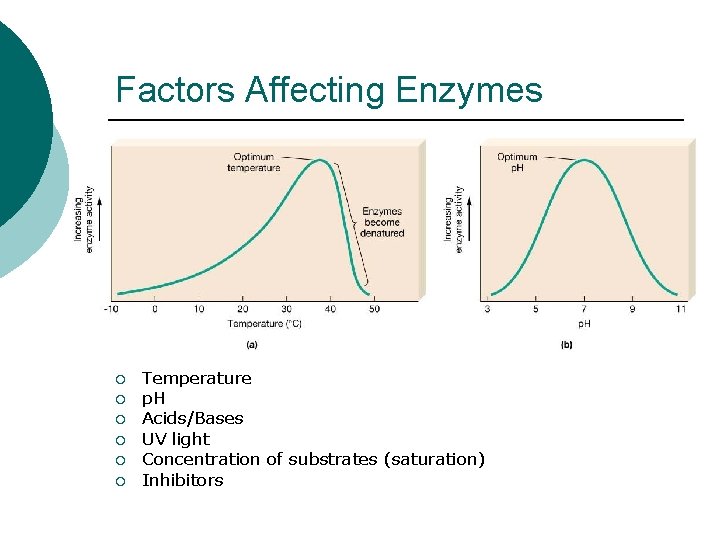
Factors Affecting Enzymes ¡ ¡ ¡ Temperature p. H Acids/Bases UV light Concentration of substrates (saturation) Inhibitors
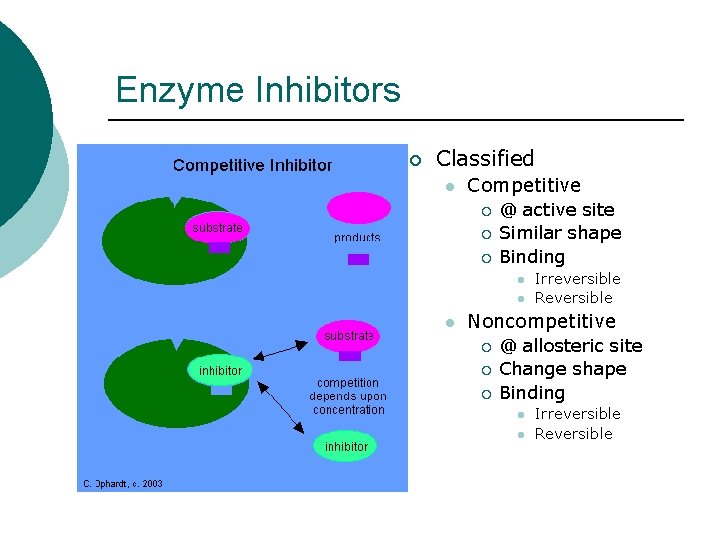
Enzyme Inhibitors ¡ Classified l Competitive ¡ ¡ ¡ @ active site Similar shape Binding l l l Irreversible Reversible Noncompetitive ¡ ¡ ¡ @ allosteric site Change shape Binding l l Irreversible Reversible
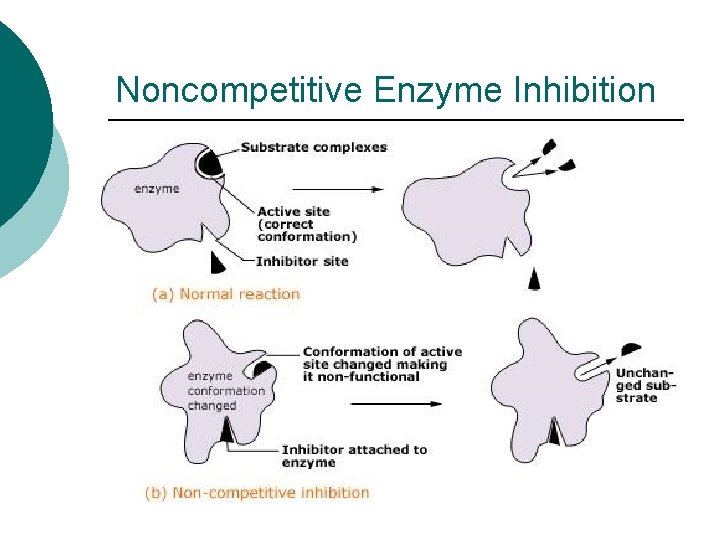
Noncompetitive Enzyme Inhibition
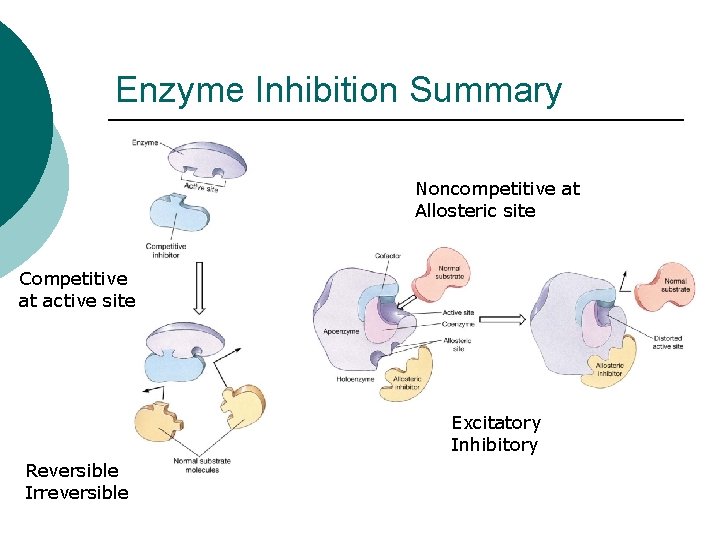
Enzyme Inhibition Summary Noncompetitive at Allosteric site Competitive at active site Excitatory Inhibitory Reversible Irreversible
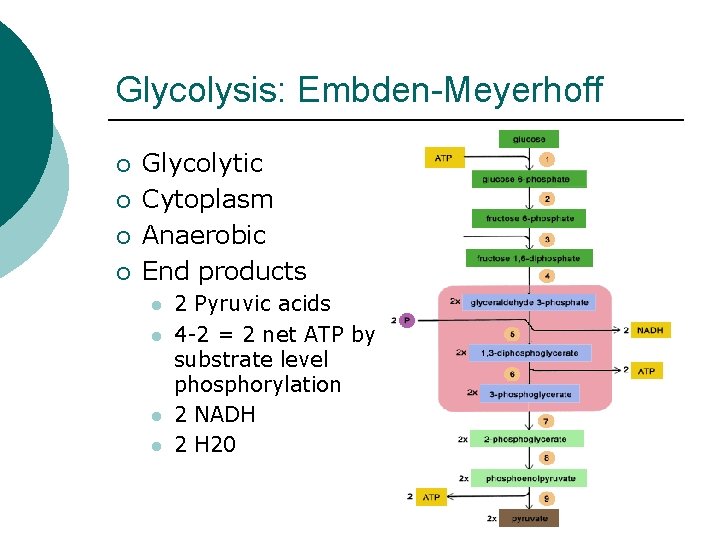
Glycolysis: Embden-Meyerhoff ¡ ¡ Glycolytic Cytoplasm Anaerobic End products l l 2 Pyruvic acids 4 -2 = 2 net ATP by substrate level phosphorylation 2 NADH 2 H 20
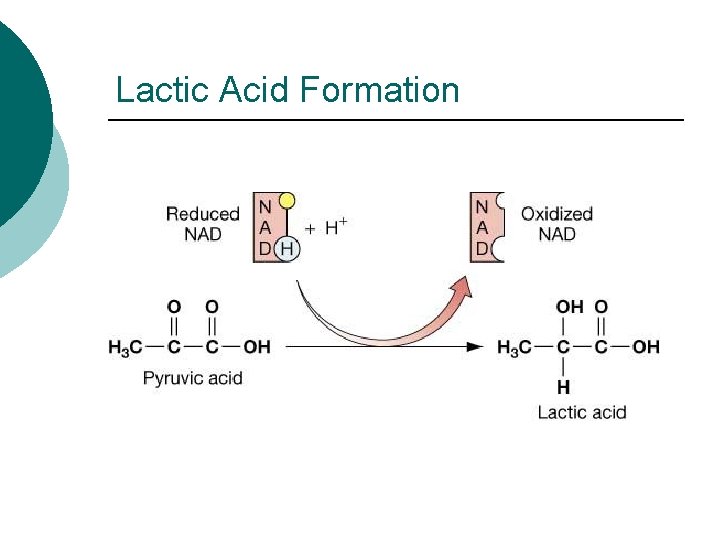
Lactic Acid Formation
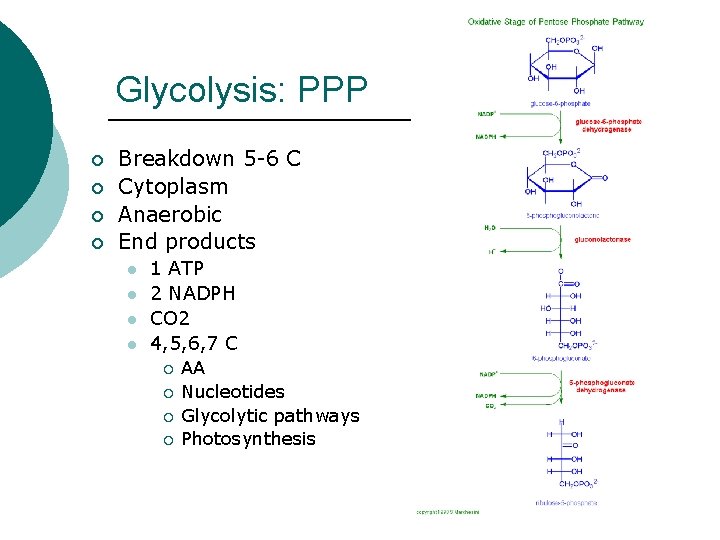
Glycolysis: PPP ¡ ¡ Breakdown 5 -6 C Cytoplasm Anaerobic End products l l 1 ATP 2 NADPH CO 2 4, 5, 6, 7 C ¡ ¡ AA Nucleotides Glycolytic pathways Photosynthesis
![Glycolysis: Entner-Duodoroff [E-D] ¡ ¡ NADP+ NADPH ¡ ¡ Glycolytic Cytoplasm Anaerobic Different enzymes Glycolysis: Entner-Duodoroff [E-D] ¡ ¡ NADP+ NADPH ¡ ¡ Glycolytic Cytoplasm Anaerobic Different enzymes](http://slidetodoc.com/presentation_image/386d3e04144fa713d77e08b85e26ac98/image-18.jpg)
Glycolysis: Entner-Duodoroff [E-D] ¡ ¡ NADP+ NADPH ¡ ¡ Glycolytic Cytoplasm Anaerobic Different enzymes l l ¡ Pseudomonas Enterococcus End products l l l 2 -1 = 1 net ATP NADPH NADH 2 Pyruvic acids H 20
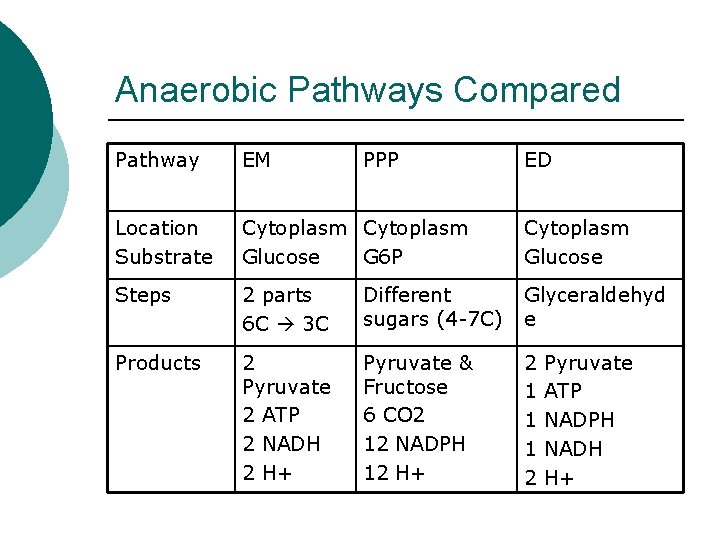
Anaerobic Pathways Compared Pathway EM PPP ED Location Substrate Cytoplasm Glucose G 6 P Cytoplasm Glucose Steps 2 parts 6 C 3 C Different sugars (4 -7 C) Glyceraldehyd e Products 2 Pyruvate 2 ATP 2 NADH 2 H+ Pyruvate & Fructose 6 CO 2 12 NADPH 12 H+ 2 1 1 1 2 Pyruvate ATP NADPH NADH H+
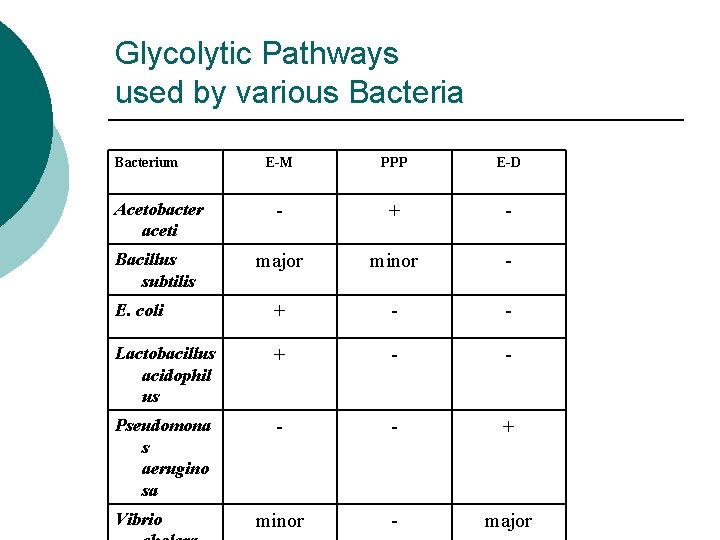
Glycolytic Pathways used by various Bacteria Bacterium E-M PPP E-D Acetobacter aceti - + - Bacillus subtilis major minor - E. coli + - - Lactobacillus acidophil us + - - Pseudomona s aerugino sa - - + minor - major Vibrio
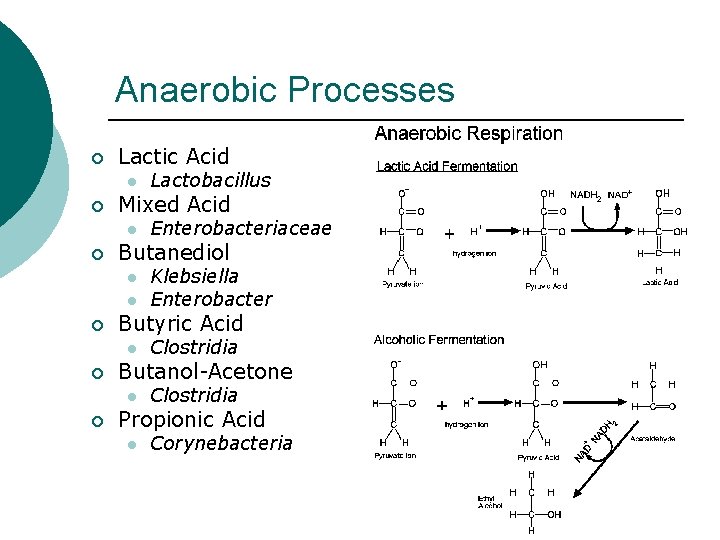
Anaerobic Processes ¡ Lactic Acid l ¡ Mixed Acid l ¡ l Clostridia Butanol-Acetone l ¡ Klebsiella Enterobacter Butyric Acid l ¡ Enterobacteriaceae Butanediol l ¡ Lactobacillus Clostridia Propionic Acid l Corynebacteria
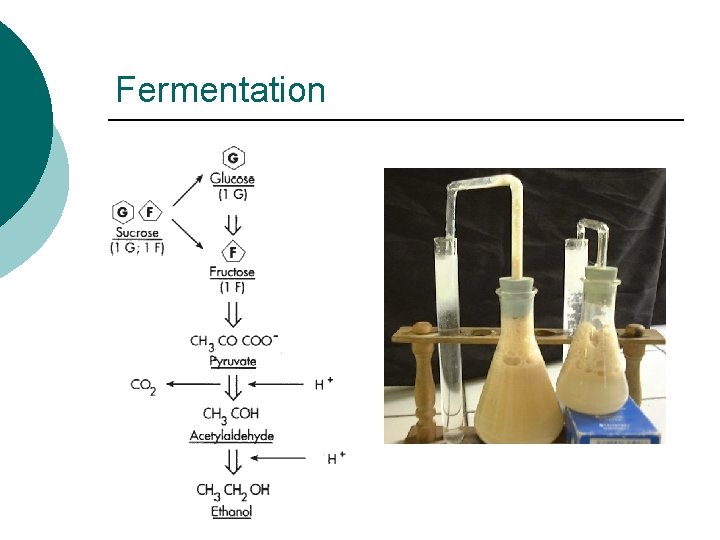
Fermentation
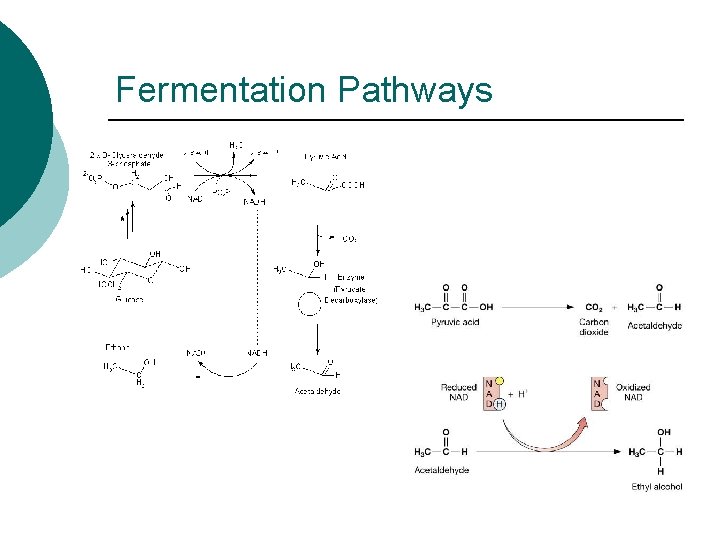
Fermentation Pathways
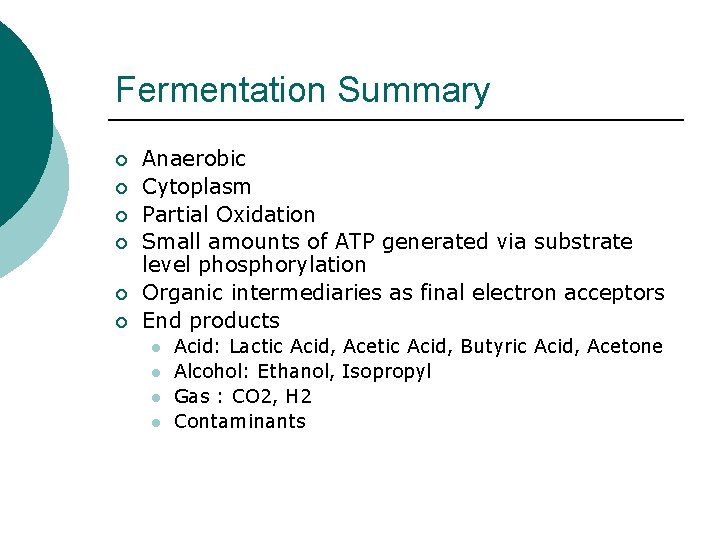
Fermentation Summary ¡ ¡ ¡ Anaerobic Cytoplasm Partial Oxidation Small amounts of ATP generated via substrate level phosphorylation Organic intermediaries as final electron acceptors End products l l Acid: Lactic Acid, Acetic Acid, Butyric Acid, Acetone Alcohol: Ethanol, Isopropyl Gas : CO 2, H 2 Contaminants
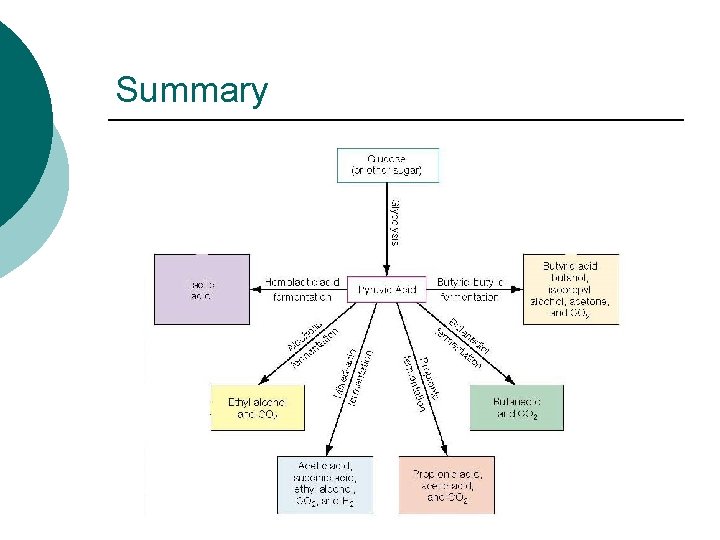
Summary
Carbohydrate Fermentation Tests
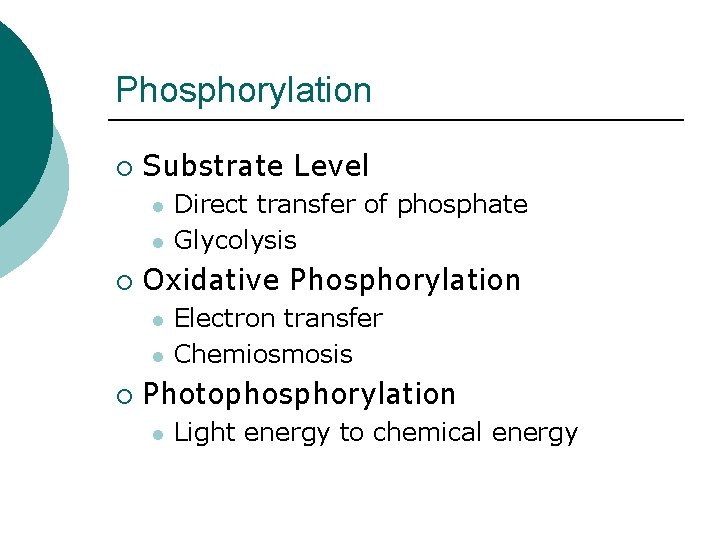
Phosphorylation ¡ Substrate Level l l ¡ Oxidative Phosphorylation l l ¡ Direct transfer of phosphate Glycolysis Electron transfer Chemiosmosis Photophosphorylation l Light energy to chemical energy
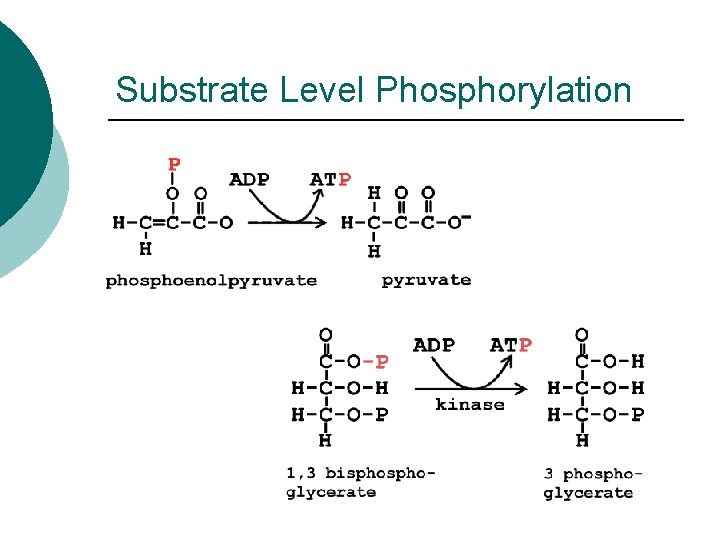
Substrate Level Phosphorylation
Aerobic Respiration
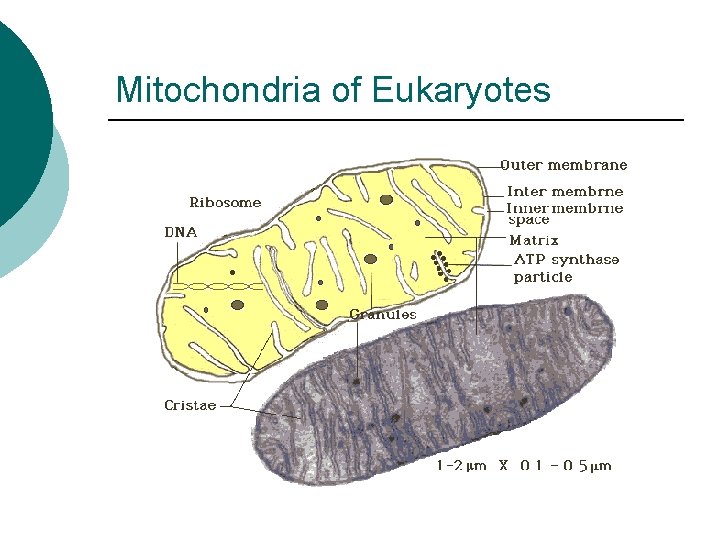
Mitochondria of Eukaryotes
![Plasma [cell] membrane Plasma [cell] membrane](http://slidetodoc.com/presentation_image/386d3e04144fa713d77e08b85e26ac98/image-31.jpg)
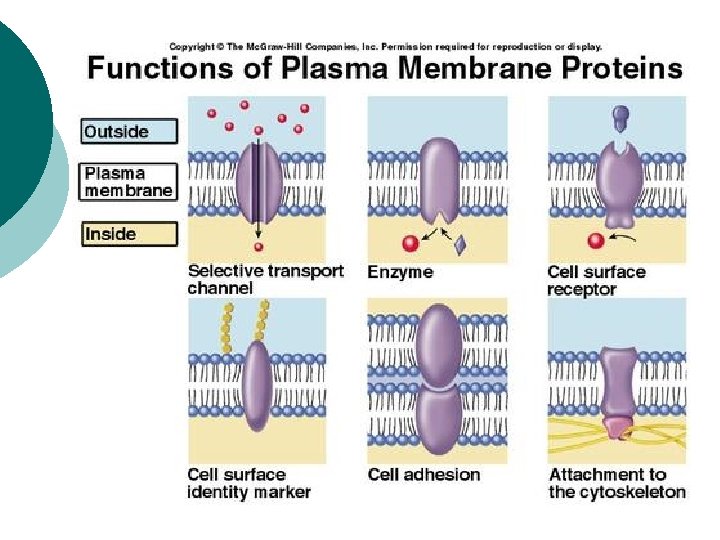
Plasma [cell] membrane
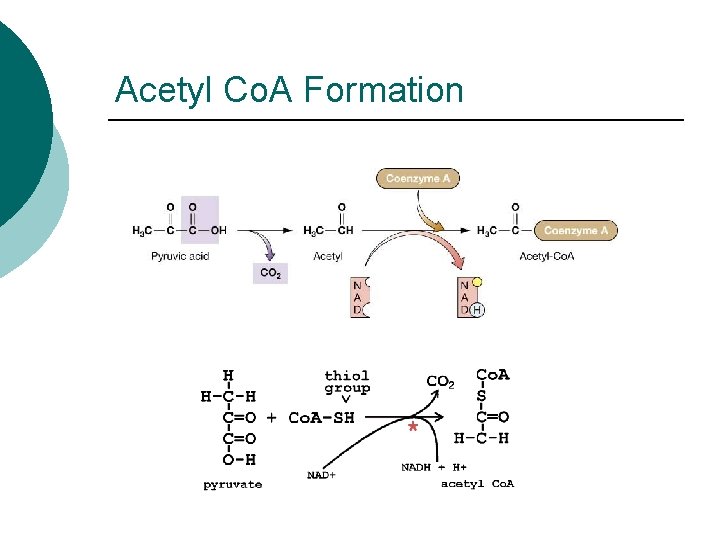
Acetyl Co. A Formation
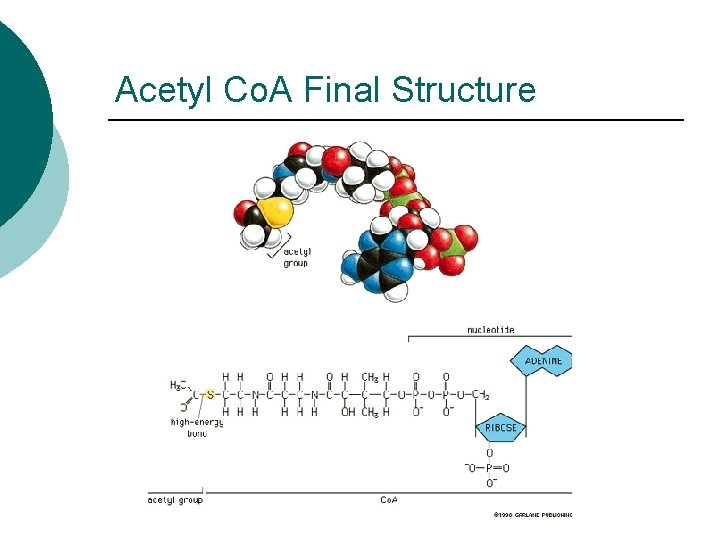
Acetyl Co. A Final Structure
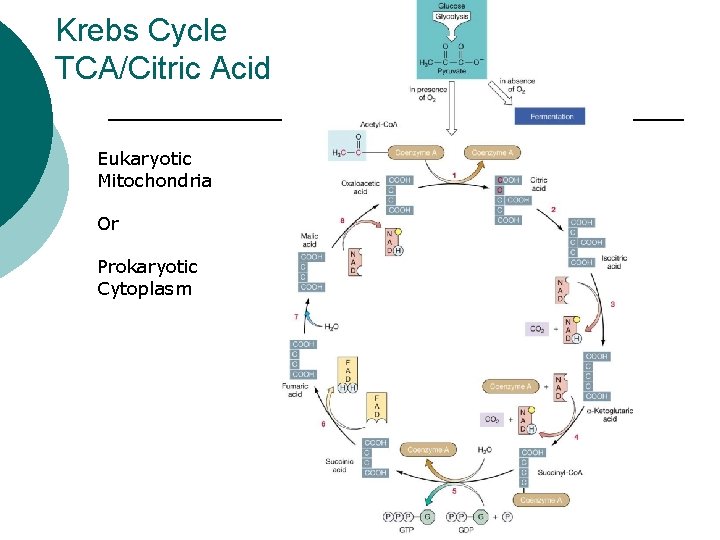
Krebs Cycle TCA/Citric Acid Eukaryotic Mitochondria Or Prokaryotic Cytoplasm
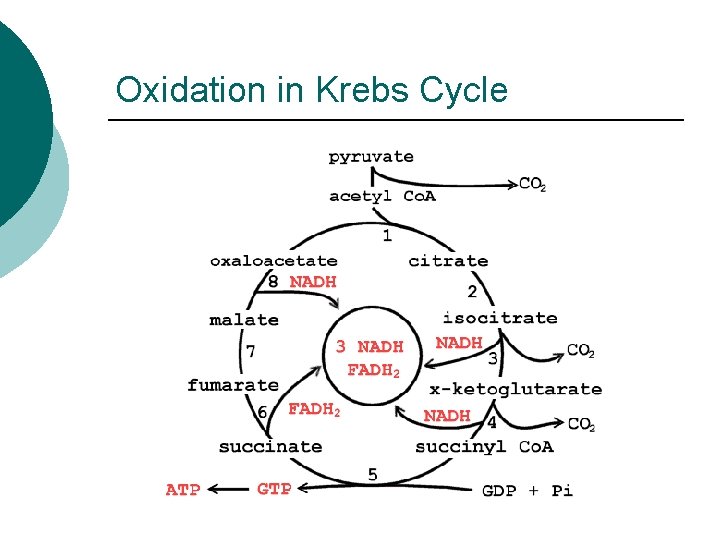
Oxidation in Krebs Cycle
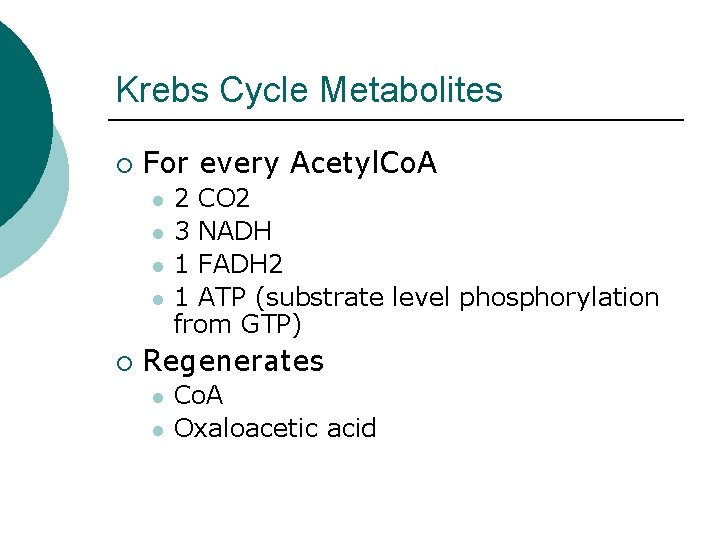
Krebs Cycle Metabolites ¡ For every Acetyl. Co. A l l ¡ 2 CO 2 3 NADH 1 FADH 2 1 ATP (substrate level phosphorylation from GTP) Regenerates l l Co. A Oxaloacetic acid

Dehydrogenation ¡ Use of hydrogen in oxidative reactions l l ¡ Removal of electron from hydrogen Carried on vitamin B derivatives Energy released is trapped in chemical bonds
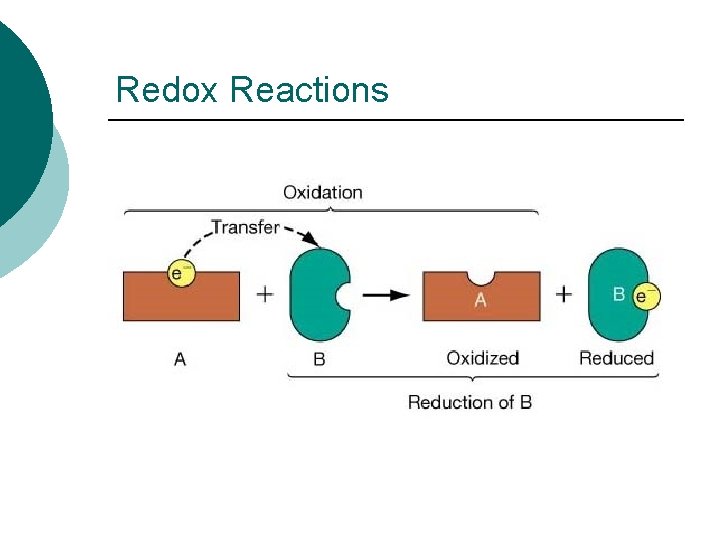
Redox Reactions
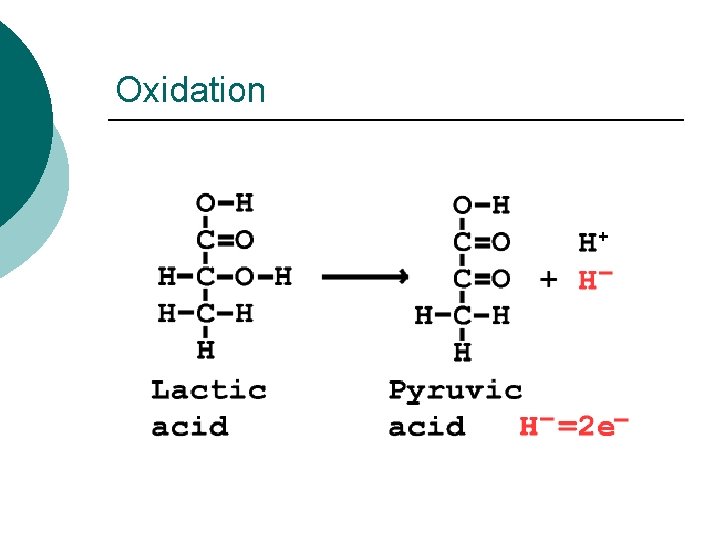
Oxidation
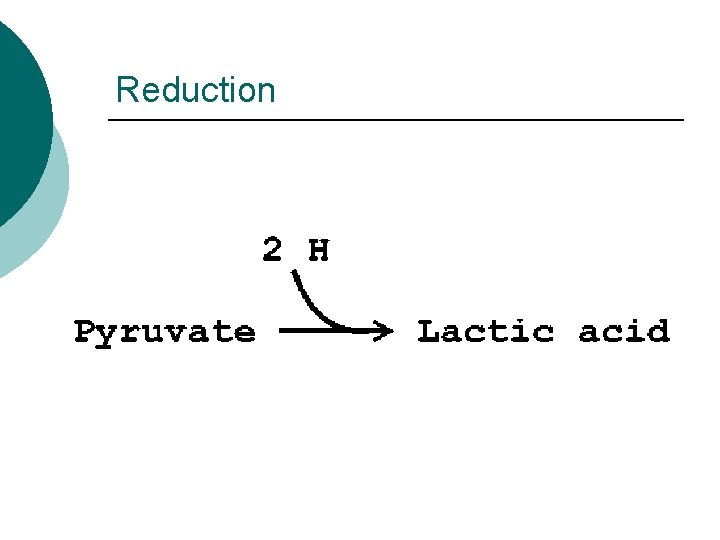
Reduction
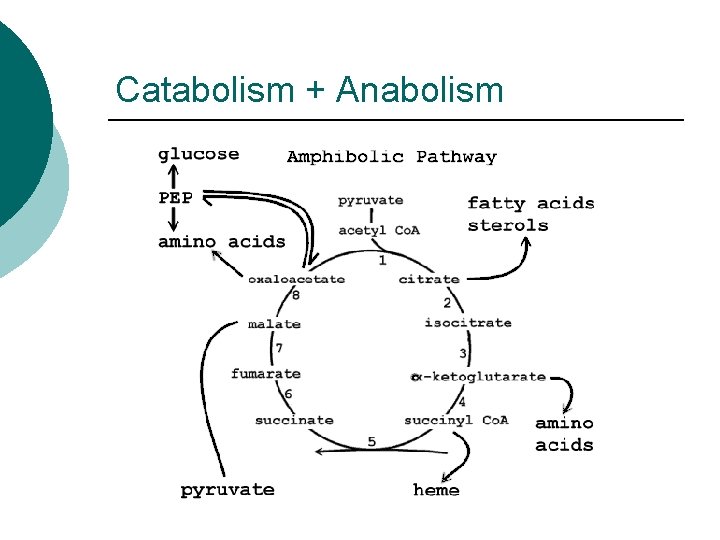
Catabolism + Anabolism
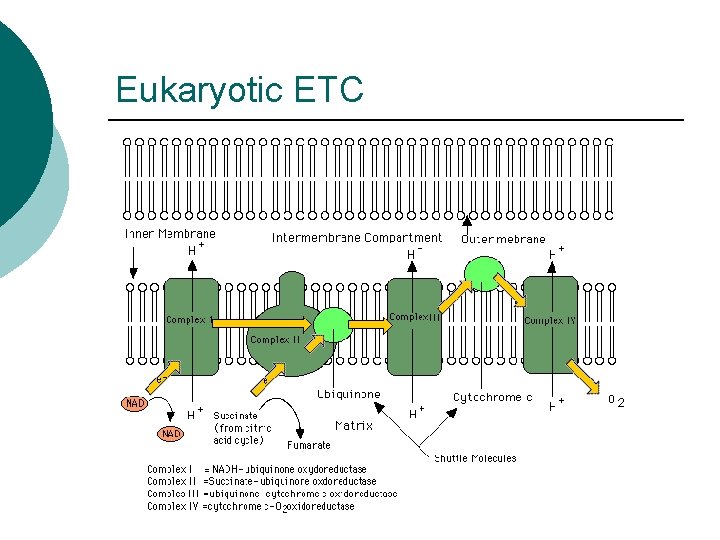
Eukaryotic ETC
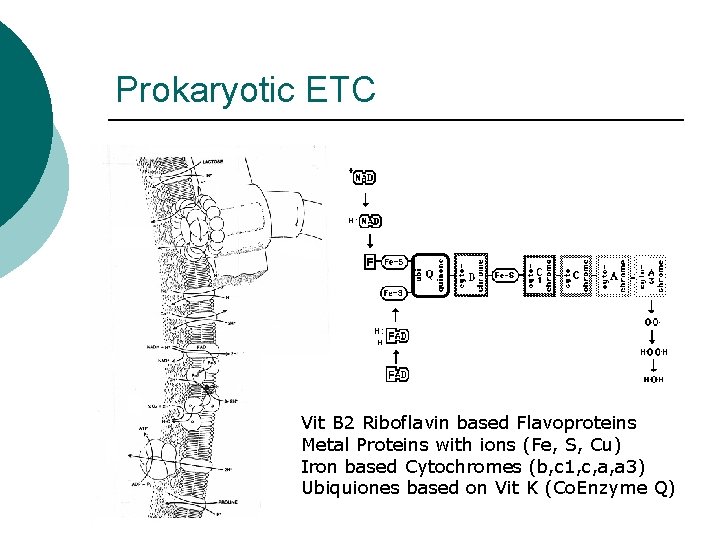
Prokaryotic ETC Vit B 2 Riboflavin based Flavoproteins Metal Proteins with ions (Fe, S, Cu) Iron based Cytochromes (b, c 1, c, a, a 3) Ubiquiones based on Vit K (Co. Enzyme Q)
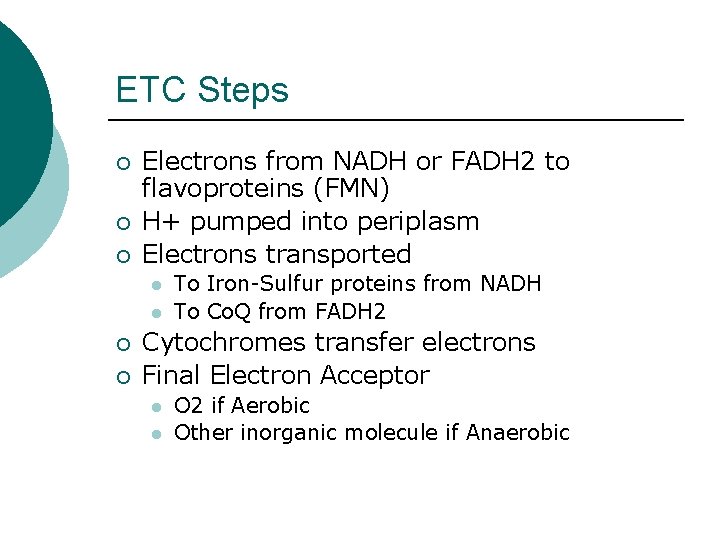
ETC Steps ¡ ¡ ¡ Electrons from NADH or FADH 2 to flavoproteins (FMN) H+ pumped into periplasm Electrons transported l l ¡ ¡ To Iron-Sulfur proteins from NADH To Co. Q from FADH 2 Cytochromes transfer electrons Final Electron Acceptor l l O 2 if Aerobic Other inorganic molecule if Anaerobic
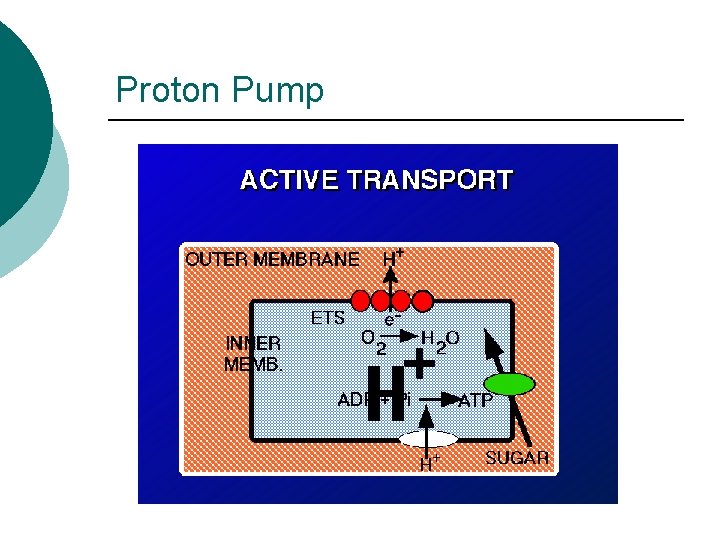
Proton Pump
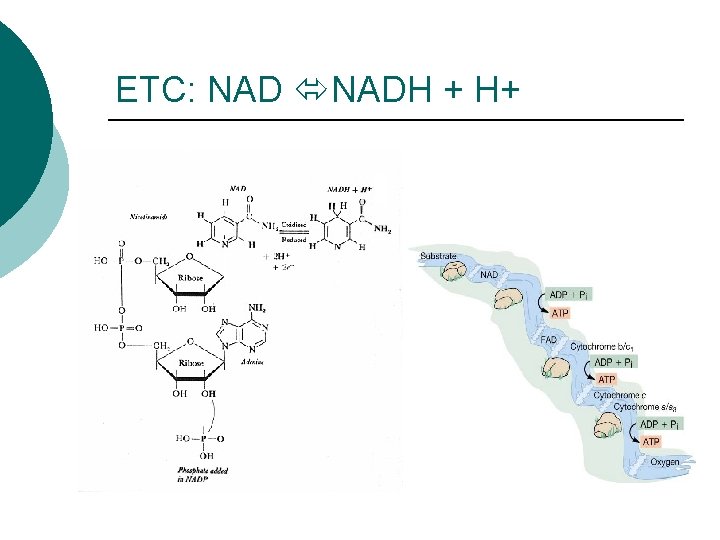
ETC: NADH + H+
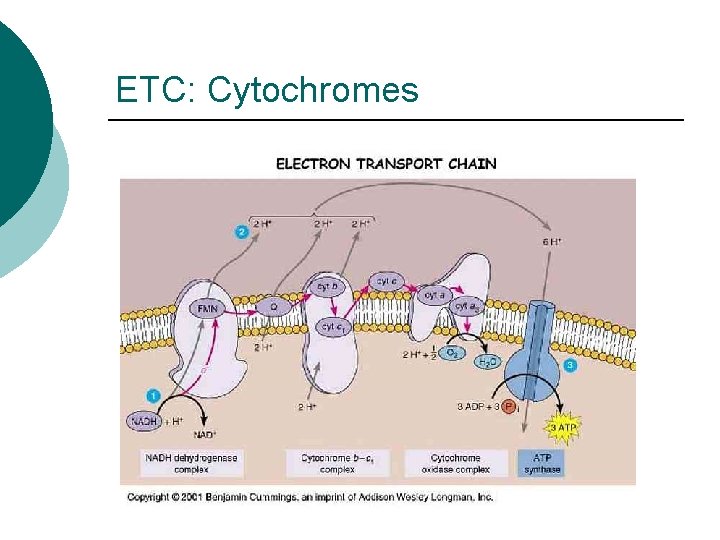
ETC: Cytochromes
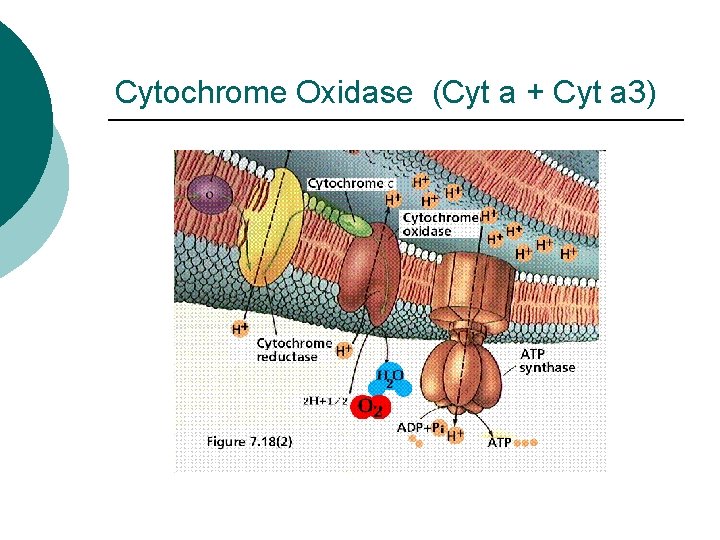
Cytochrome Oxidase (Cyt a + Cyt a 3)
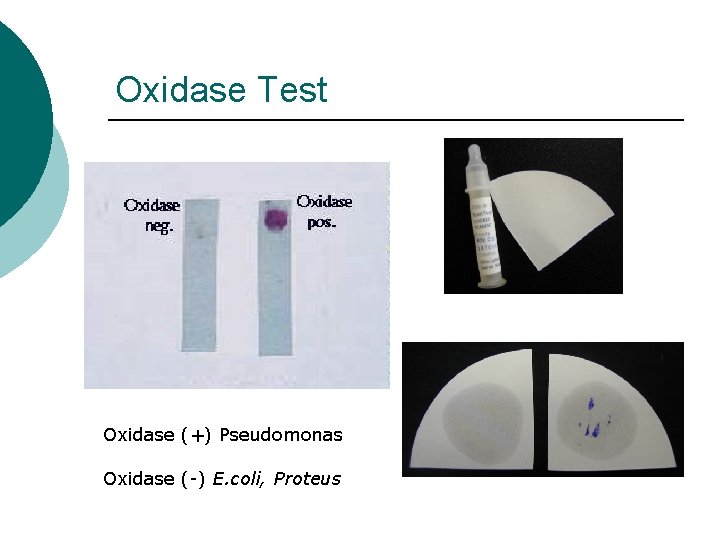
Oxidase Test Oxidase (+) Pseudomonas Oxidase (-) E. coli, Proteus
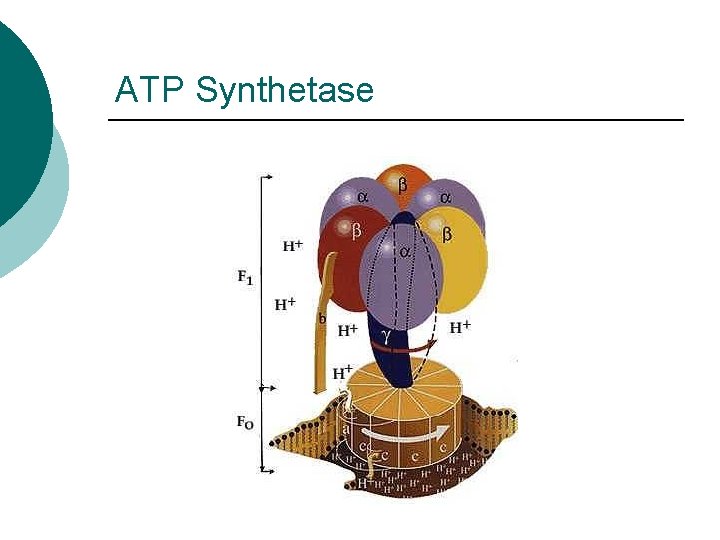
ATP Synthetase
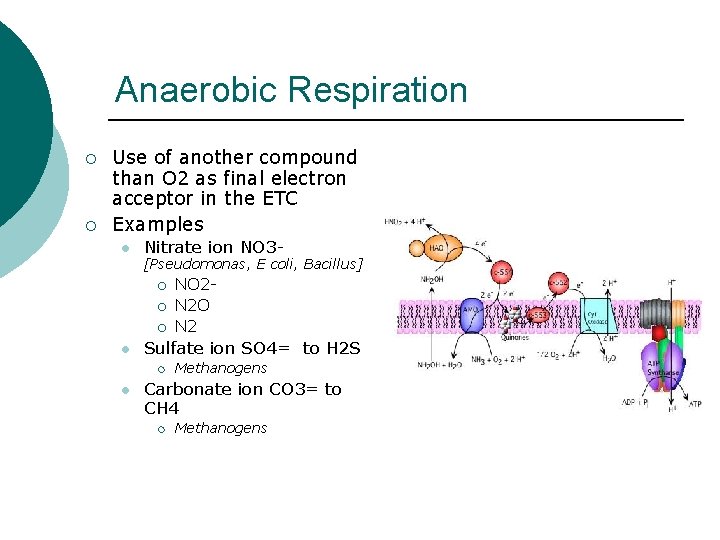
Anaerobic Respiration ¡ ¡ Use of another compound than O 2 as final electron acceptor in the ETC Examples l Nitrate ion NO 3 - [Pseudomonas, E coli, Bacillus] ¡ ¡ ¡ l Sulfate ion SO 4= to H 2 S ¡ l NO 2 N 2 O N 2 Methanogens Carbonate ion CO 3= to CH 4 ¡ Methanogens
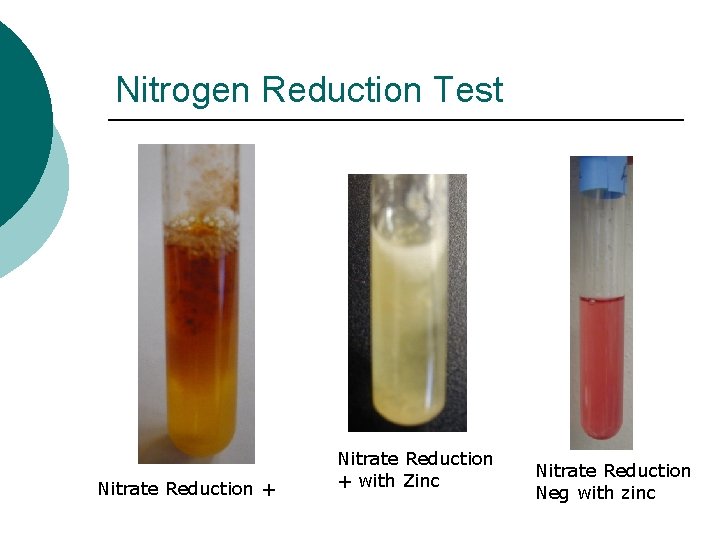
Nitrogen Reduction Test Nitrate Reduction + with Zinc Nitrate Reduction Neg with zinc
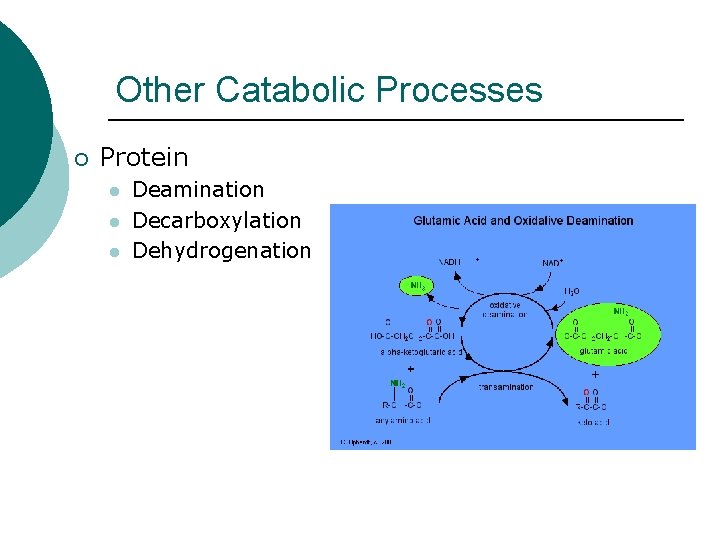
Other Catabolic Processes ¡ Protein l l l Deamination Decarboxylation Dehydrogenation
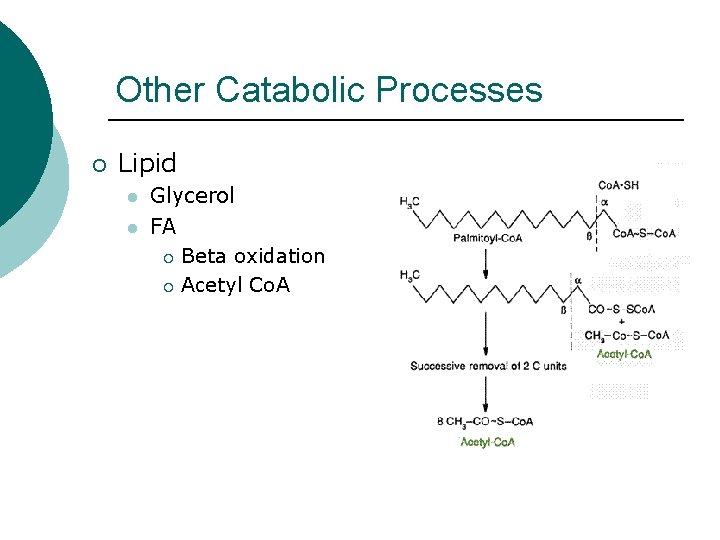
Other Catabolic Processes ¡ Lipid l l Glycerol FA ¡ Beta oxidation ¡ Acetyl Co. A
Biosynthesis ¡ Polysaccharide l l l ¡ Lipid l ¡ Glycerol + FA AA l ¡ Keto acids AA Glycerol from FA Keto acids + NH 2 Nucleotides l l Nitrogen bases from Keto acids + NH 2 5 C sugars from alternate CH 20 Metabolism
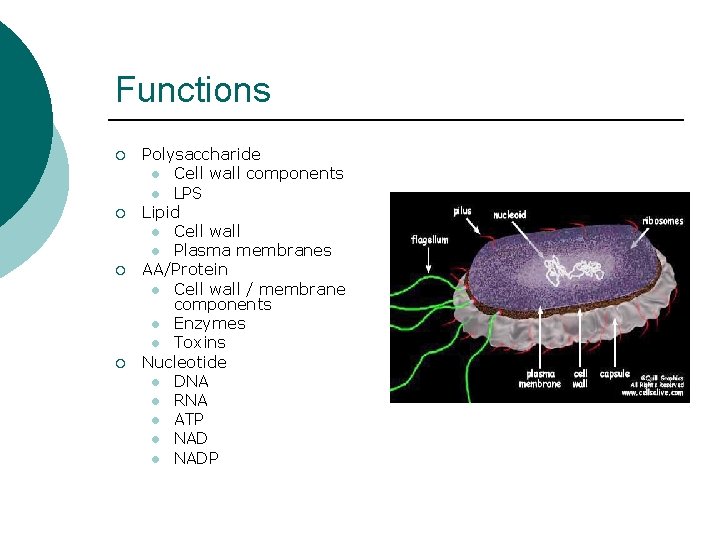
Functions ¡ ¡ Polysaccharide l Cell wall components l LPS Lipid l Cell wall l Plasma membranes AA/Protein l Cell wall / membrane components l Enzymes l Toxins Nucleotide l DNA l RNA l ATP l NADP

Protein Synthesis and Enzyme Regulation
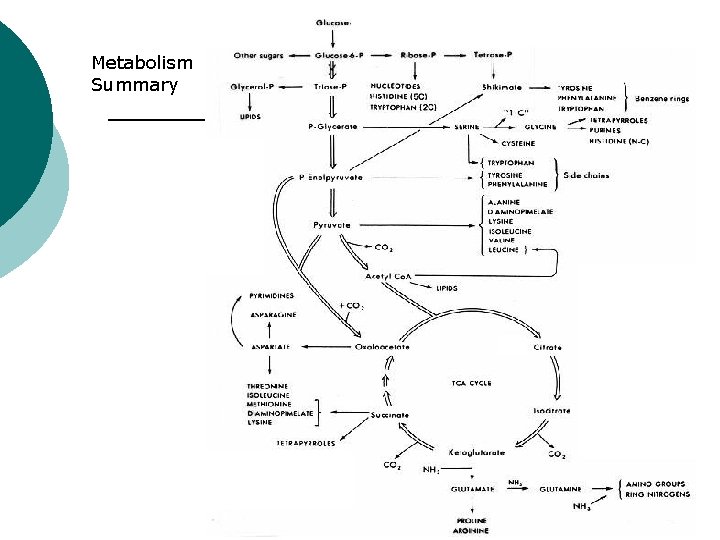
Metabolism Summary

Questions?
- Slides: 60