Microbial Indicator Concepts and Purposes The types of

Microbial Indicator Concepts and Purposes • The types of pathogens that can contaminate water, food, air and other environmental media are diverse and there are many different ones. • Measuring all of these pathogens on a routine basis for determining presence or absence or acceptable concentration is not possible. – Methods are not available to recover and measure some of them, – Methods are available for other pathogens, but they are technically demanding, some are slow to produce results and their costs are high. • The alternative is to measure something other than a pathogen that is indicative of contamination, predicts pathogen presence and estimates human health risks.
What is Measured as Microbial Indicators and Why? • Microbial indicators have been used for more than 100 years (since late 1800 s) to detect and quantify fecal contamination in water, food and other samples – Concerns were for bacteria causing water- and foodborne illness, such as: • Salmonella typhi: the cause of typhoid or enteric fever • Vibrio cholerae: the cause of cholera • Shigella dysenteriae and other Shigella species: dysentery • Focus was and still is on detecting primarily human (or maybe animal) fecal contamination as the source of these and other enteric bacterial pathogens • Detect fecal contamination by measuring: – common enteric bacteria residing in the gut and shed fecally – Chemicals associated with the gut or with anthropogenic fecal contamination

What is Measured as Microbial Indicators and Why? • Microbial indicators also are used to indicate other conditions unrelated to fecal contamination, such as : – Food spoilage bacteria and molds – Excessive microbial growth in water • Causing appearance, taste and odor problems: – “red water” from iron biofouling – Blooms of algae and cyanobacteria (bluegreen algae) » Some of the organisms harbor or release toxins (“red tides”)

What is Measured as Microbial Indicators and Why? • Airborne contamination: – From wet buildings: molds and actinomycetes – From industrial processes: • bacterial endotoxins from cotton dust, solid waste and other sources • Microbial allergens from manufacturing processes (aerosols and dusts) – total airborne microbe concentrations • In health care facilities • In “clean room” manufacturing environments for electronics and pharmaceuticals • From composting operations – Salivary bacteria from dentistry activities
Pathogen Detection and Monitoring • Pathogen detection – technically demanding, – often tedious, – slow to produce results, – Often unreliable – expensive. • Done routinely in the health care field (clinical diagnostic microbiology): – often essential to patient treatment and care. – provides national surveillance of infectious disease epidemiology

Pathogen Analysis, Monitoring and Surveillance • Until recently, rarely done for managing food quality – Salmonella and E. coli O 157: H 7 are now monitored in meat and poultry; Listeria monocytogenes monitoring also being done • Rarely done for monitoring or managing water quality – pathogen occurrence surveys and special studies: • survey (18 months) for Giardia, Cryptosporidium and enteric viruses in larger drinking water supplies using surface water sources: ICR (Information Collection Regulation) • survey for enteric viruses in ground water sources of drinking water (data base for Ground Water Disinfection Rule) – investigation of waterborne outbreaks and pilot/in-plant studies – Pathogen monitoring sometimes done for biosolids (Class A) • Salmonella, viable Ascaris ova, culturable enteric

Sampling Considerations What we want: • Fast • Sensitive • Specific • Easy to Perform • Reliable (Accurate/Precise) • Compatible with Downstream Detection What do we have? ? ?

The Challenge of Environmental Sampling for Pathogens • Variation in microbe type and distribution • Low microbe numbers: need to concentrate them • Non-random distribution and physical state of microbes of interest: aggregated, particleassociated, embedded, etc. • Volume considerations • Environmental factors may inhibit or interfere with downstream detection • Separate them from interfering and excess other material

Detection of Pathogens in The Environment • • Three main steps: (1) recovery and concentration, (2) purification and separation, and (3) assay and characterization.

Aerosol Sampling • Impactor – Anderson single and multistage sampler – Slit sampler – Rotary arm sampler • Impinger – AGI sampler – Biosampler (SKC) sampler • Filters – IOM/Button filter sampler – Foam plug filter sampler • Centrifugal – Cyclone sampler – Centrifugal sampler • Precipitators – Electrostatic precipitator – Condensation trap • Hybrid

Bioaerosol Sampling John Scott Meschke 4225 Roosevelt Way NE, suite 2338 jmeschke@u. washington. edu 206 -221 -5470

Bioaerosols • A collection of aerosolized biological particles (e. g. microbes, by-products of living organisms) capable of eliciting diseases that may be infectious, allergic, or toxigenic with the conditions being acute or chronic • Size range 0. 02– 100 micrometers (typically 2 -10 microns size range of most concern) • Composition of the particles varies with source and environmental conditions • Particles can contain varying amounts of water • Some are colloidal particles of soil, vegetation, other material • Viruses, bacteria and fungi (spores and hyphae) in aerosols due to small size • Many protozoa too large to remain airborne

Examples: Agents of Respiratory Infections Viruses: influenza, measles (rubeola), chickenpox (herpes varicella‑zoster) and rhinoviruses (colds); Hantavirus (from a rodent; mouse) Bacteria: Legionella spp. , tuberculosis and other mycobacteria (Mycobacterium spp. ), anthrax (Bacillus anthracis), and brucellosis (Brucella spp. ). Fungi: diseases: histoplasmosis, cryptococcosis, blastomycosis, coccidiodomycosis, and aspergillosis Protozoans: Pneumocystis carinii pneumonia; prevalent in immunodeficient hosts such as
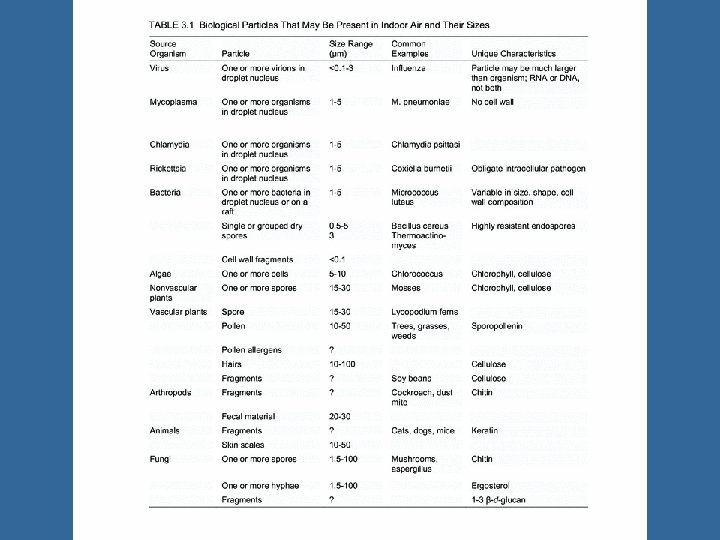
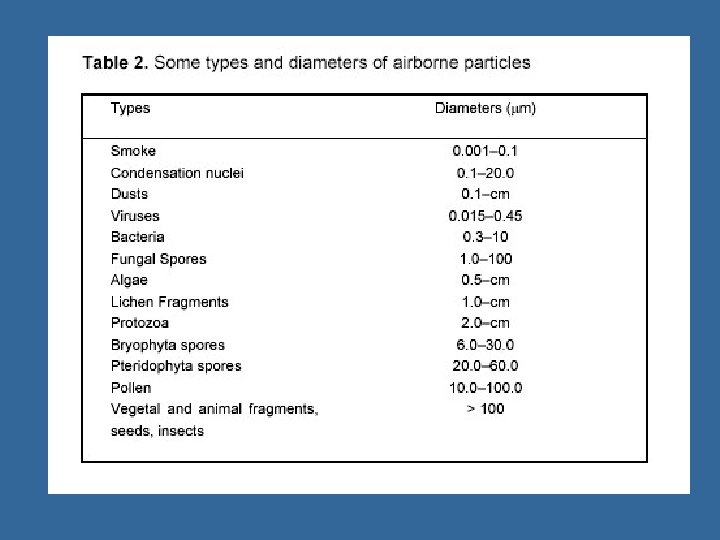

Reservoirs and Amplifiers of Airborne Microbes Wide range, overall Depends on the microbe – humans, – animal, – soil – dust – water – air Amplifiers: • Places where microorganisms multiply or proliferate. • Most reservoirs are potential amplifiers.

Airborne Microbes and their Reservoirs Viruses: • Mostly humans but some animals • Some rodent viruses are significant: ex: Lassa Fever Virus and Hantavirus. Bacteria: • • Humans (TB & staphylococci), other animals (brucella and anthrax), water (Legionella) soil (clostridia). Fungi: • • • soil and birds (Cryptococcus and Histoplasma) dead plant material wet surfaces (wood and other building materials) indoor air (mycotic air pollution) stagnant water for the opportunistic fungi (e. g. , Aspergillus sp. ).

Disseminators • Devices causing microbes to enter airborne state or be aerosolized; often the reservoir or amplifier. • Any device able to produce droplets and aerosols: – Humans and other animals: coughs and sneezes, esp. – Mechanical ventilation systems – Nebulizers and vaporizers – Toilets (by flushing) – Showers, whirlpools baths, Jacuzzi, etc. – Wet or moist, colonized surfaces (wet walls and other structures in buildings) – Environments that are dry and from which small particles can become airborne by scouring or other mechanisms: • Vacuuming or walking on carpets and rugs • Excavation of contaminated soil • Demolition of buildings
Bioaerosol Samplers • Numerous sampler types • Some adapted from dust or particle samplers • Some designed specifically for microbes • Few specifically for non-microbial bioaerosols (e. g. endotoxin), but generally thought samplers used for microbe collection are adaptable
Bioaerosol Samplers • Gravitational samplers (e. g. settle plates) – No special equipment only coated microscope slide, agar plates, etc. – Passive (non-volumetric), relies on collection of particles by gravity settling – Oversamples for larger particles – Poor for collection in turbulent air; affected by turbulent deposition or shadowing
Inertial Bioaerosol Samplers • Allow collection of particles by size selective sampling • Includes impactors, sieves, stacked sieves • Relies on particle tendency to deviate from air flow streamlines due to inertia • Particles deposited to solid or semi-solid surface

Spore Traps • E. g. Hirst, Burkhard, Air-o-cell, Allergenco • Initially designed for fungal spore and pollen • Sample at 10 -20 Liters/minute • Particles impacted on to coated glass slide or adhesive tape • Advantages: non-selective, direct analysis after collection • Disadvantages: may mask problem species, does not assess viability
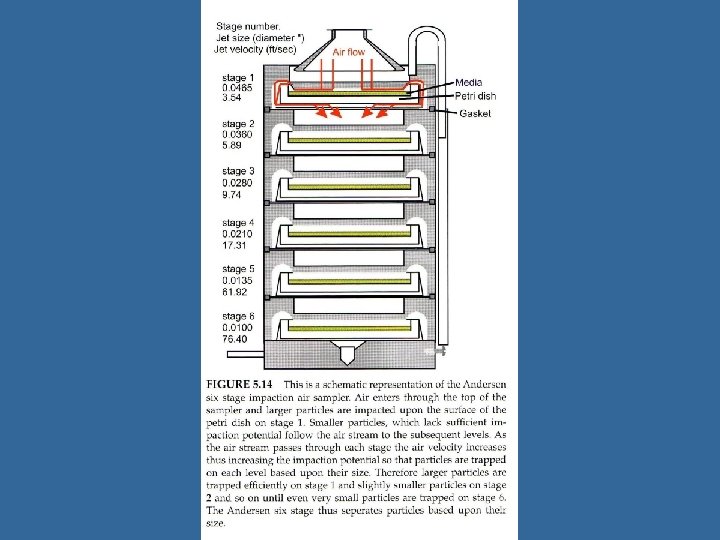
Impactors • Similar to spore trap, but collection on slide or agar plates • Several designs tend to undersample smaller particles; particle bounce can also be an issue • Used at air flows of 10 -30 Liters/minute • Types: – Single Stage or Multistage (e. g. Anderson) – Rotary arm samplers (e. g. Rotorod, Mesosystems BT 550) – Slit to agar samplers – Sieve Samplers and Stacked Sieves (e. g. SAS)
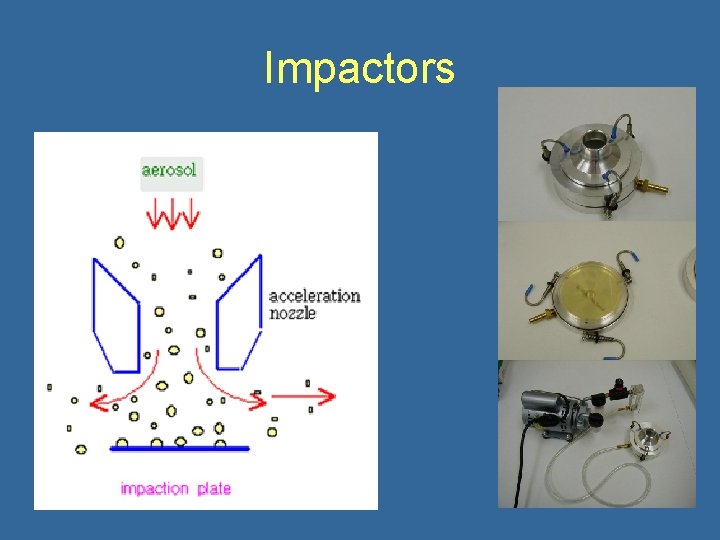
Impactors
Impingers • Air drawn through liquid (e. g. water, broth, mineral oil), particles removed by impingement • Allows dilution • Problems with pass through, particle bounce, bubbling, evaporation of liquid loss of viability • Inlet efficiency decreased for particles above 10 microns • Sampling rate 0. 1 -15 liters/minute (12. 5 for AGI 30) • Types: – – AGI Biosampler Shipe Multistage
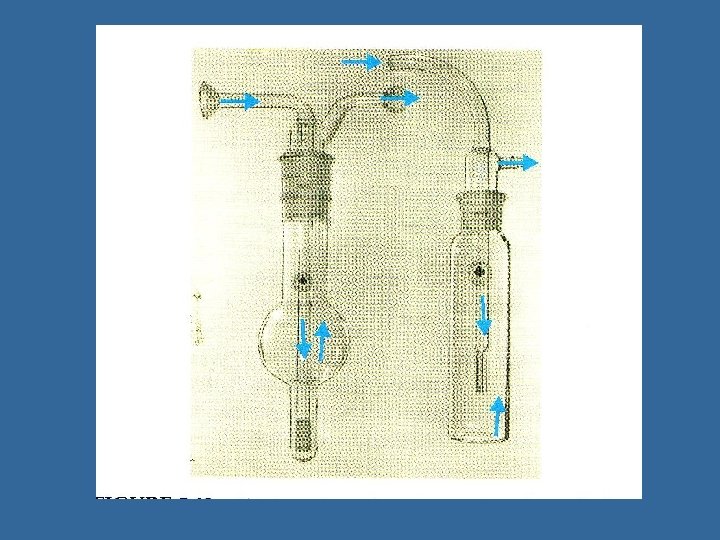
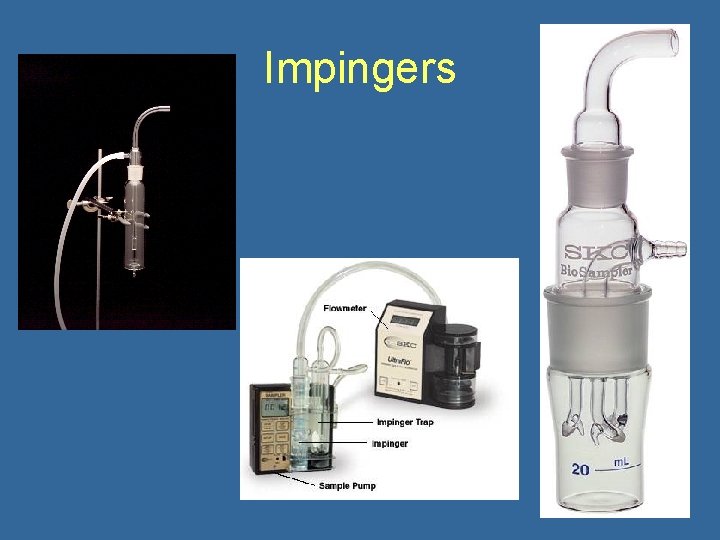
Impingers
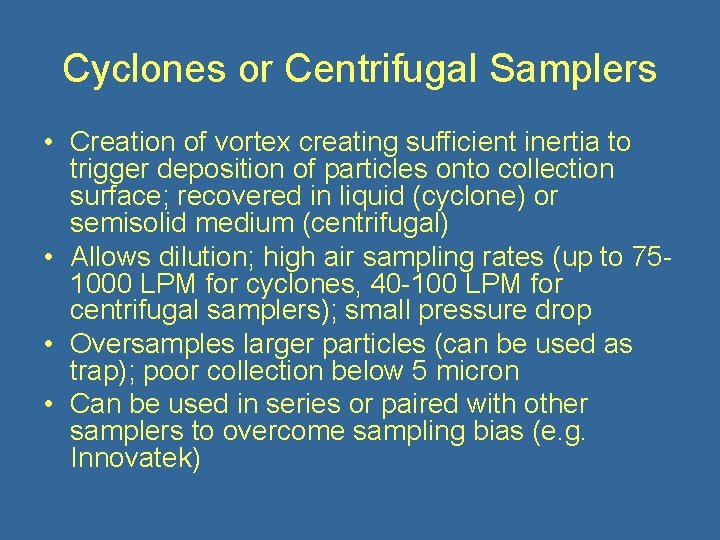
Cyclones or Centrifugal Samplers • Creation of vortex creating sufficient inertia to trigger deposition of particles onto collection surface; recovered in liquid (cyclone) or semisolid medium (centrifugal) • Allows dilution; high air sampling rates (up to 751000 LPM for cyclones, 40 -100 LPM for centrifugal samplers); small pressure drop • Oversamples larger particles (can be used as trap); poor collection below 5 micron • Can be used in series or paired with other samplers to overcome sampling bias (e. g. Innovatek)
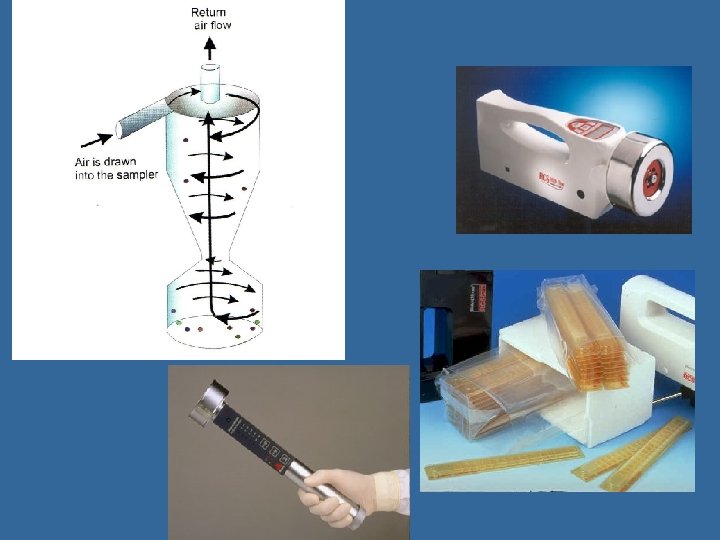

Large Volume Aerosol Samplers • Biocapture BT 550 (Mesosystems) – Rotary arm impactor, liquid collection – 150 L/min (~15 min) • Bioguardian (Innovatek) – Wet-walled multi cyclone, w/centrifugal impactor for removal of large particles – 100 -1000 L/min (1 min-12 hours) • Spincon (Sceptor) – Centrifugal wet concentrator, w/cyclonic preseparation – 450 L/min (5 min-6 hours)

Aerosol Samplers
Non-Inertial Samplers • E. g. Filtration, Electrostatic Precipitation, thermal precipitators, and Condensation traps • Do not rely on inertia of particles for operation, thus less reliant on particle size (less particle size bias)
Filtration • Simple equipment requirements • Adaptable to personal sampling • Less particle size bias (allows large and small particle collection; dependent on inlet size/shape) • Continuous sampling over extended period • Wide variety of sampling rates • However, problems with desiccation leading to reduced viability and difficulties with particle recovery efficiencies

Filter Media • Fiborous- mesh of material whose fibers are randomly oriented (creating nominal pore size); depth filter entrainment – Glass fiber (works for proteinaceous bioaerosols) • Membrane- a gel with interconnected pores of uniform size (absolute pore size); depth filter entrainment – Cellulose esters (commonly used for water and other liquids for microbe concentration), PVC, PTFE, nylon, gelatin • Flat disc or etched membranes- defined holes or pores (absolute pore size); surface collection – Silver, aluminum oxide, polycarbonate (most commonly filter media for collection of microbes from air)

Filters

Electrostatic Precipitators • Particles removed from air stream by electrical rather than inertial forces • Low pressure drop; low power; capable of large volume sampling and high rates • Draws air across high voltage field or corona discharge inducing charge; surface collection • Can be effective for very small particles, as well as larger ones • Problem with ozone production; loss of viability • Examples– LVAS – LEAP
Thermal Precipitation and Condensation Traps • Thermal precipitation – Not commonly used – Based on Thermophoretic motion – Air passed between two plates (one heated and one cooled); particles collected on cooler plate • Condensation trap – Relies on manipulation of relative humidity – Bioaerosol used as condensation nuclei – Particles collected by settling

Recovery from Air • Factors that will affect the recovery of microbes from air samples: – Sampling Rate – Environmental Factors may reduce sampling efficiency (e. g. Swirling winds) – Sampling Time – Organism Type and Distribution – Particle Size and Distribution – Target of detection method to be utilized – Sampler Choice • Collection efficiency • Recovery efficiency • Particle Size Bias

Recovery from Air • Factors that will affect the recovery of microbes from air samples: – Sampling Rate and Sampling Time (sampled volume) – Concentration factor – Environmental Factors may reduce sampling efficiency (e. g. Swirling winds) – Organism Type and Distribution (need for replication) – Target of detection method to be utilized – Sampler Choice • • • Collection efficiency (d 50) Retention efficiency Recovery efficiency Particle Size Bias Loss of viability – Sampler Calibration
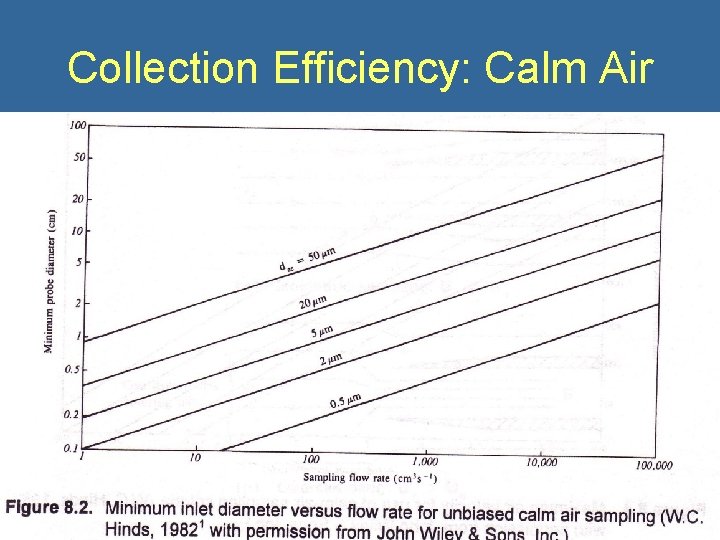
Collection Efficiency: Calm Air
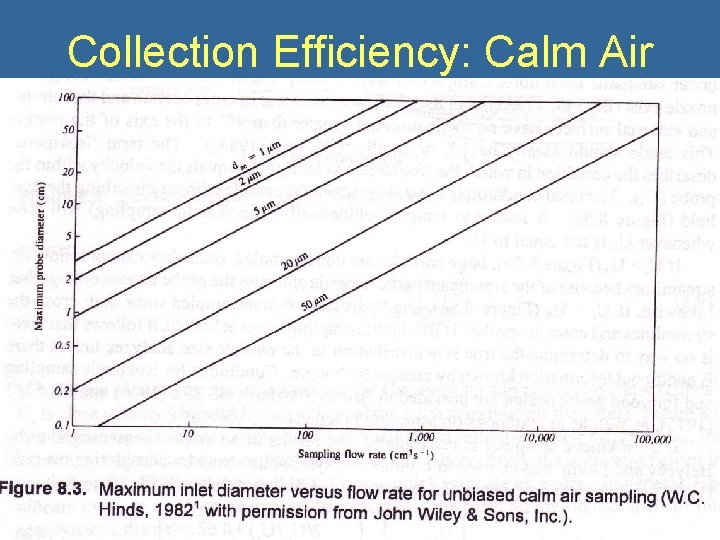
Collection Efficiency: Calm Air
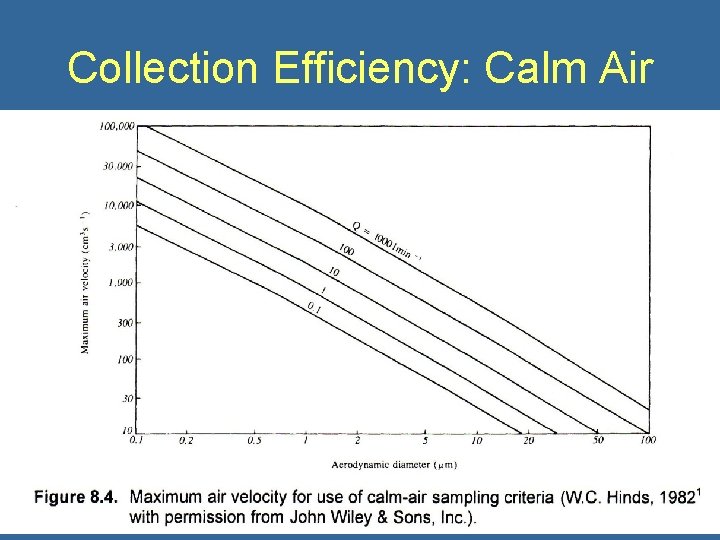
Collection Efficiency: Calm Air
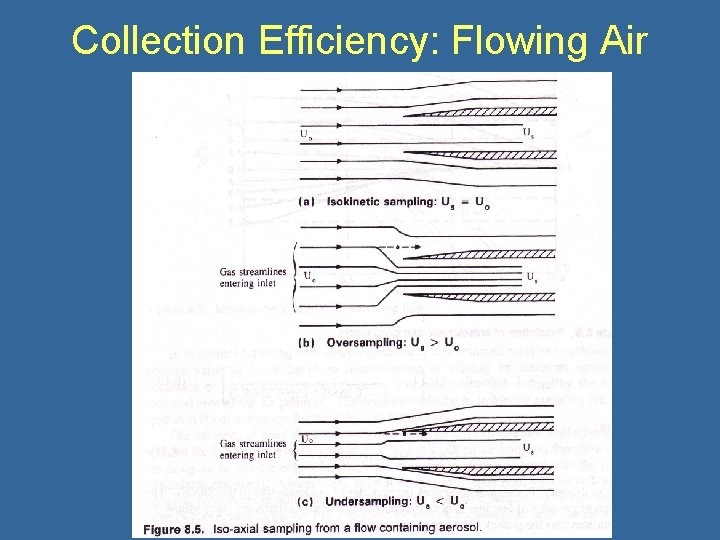
Collection Efficiency: Flowing Air

Sample Line Losses • To minimize make as short as possible, minimize angles

Separation and Purification
Separation and Purification Methods • Purification, separation and secondary concentration of target microbes in primary sample or sample concentrate – Separate target microbes from other particles and from solutes – Reduce sample size (further concentrate)

Separation/Purification Methods • Variety of physical, chemical and immunochemical methods: – Sedimentation and flotation (primarily parasites) – Precipitation (viruses) – Filtration (all classes) – Immunomagnetic separation or IMS (all classes) – Flow cytometry (bacteria and parasites); an analysis, too
Secondary Concentration and Purification • • • PEG (polyethylene glycol) Organic Flocculation IMS (Immunomagnetic separation) Ligand capture BEa. Ds (Biodetection Enabling Device) Capillary Electrophoresis Microfluidics Nucleic Acid Extraction Spin Column Chromatography Floatation Sedimentation Enrichment
Chemical Precipitation Methods • Viruses: precipitate with polyethylene glycol or aluminum hydroxide – resuspend PEG precipitate in aqueous buffer – dissolve aluminum floc in dilute acid solution – both have been used as second-step concentration and purification methods • Parasites: precipitate with calcium carbonate – dissolve precipitate in dilute sulfamic acid

Other Recovery and Concentration Methods • Minerals, such as iron oxide and talc; used to adsorb viruses • Synthetic resins: ion exchange and adsorbent • Other granular media: glass beads and sand Less widely used; less reliable, cumbersome; uncertain elution, desorption, exchange efficiencies
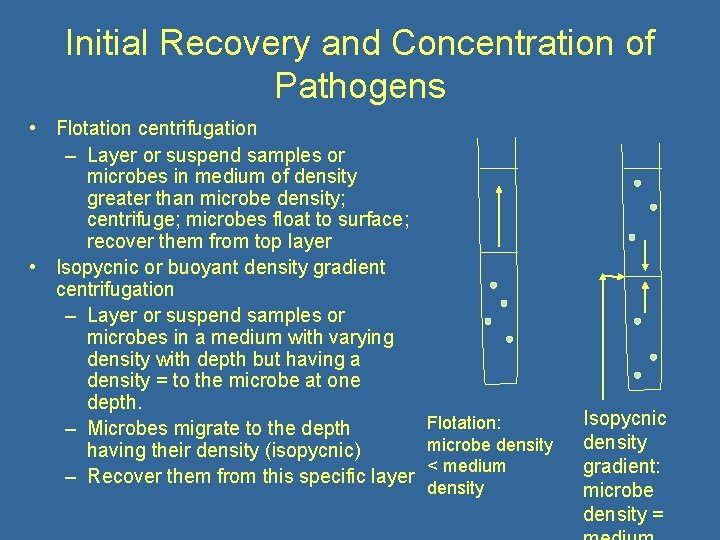
Initial Recovery and Concentration of Pathogens • Flotation centrifugation – Layer or suspend samples or microbes in medium of density greater than microbe density; centrifuge; microbes float to surface; recover them from top layer • Isopycnic or buoyant density gradient centrifugation – Layer or suspend samples or microbes in a medium with varying density with depth but having a density = to the microbe at one depth. Flotation: – Microbes migrate to the depth microbe density having their density (isopycnic) < medium – Recover them from this specific layer density Isopycnic density gradient: microbe density =
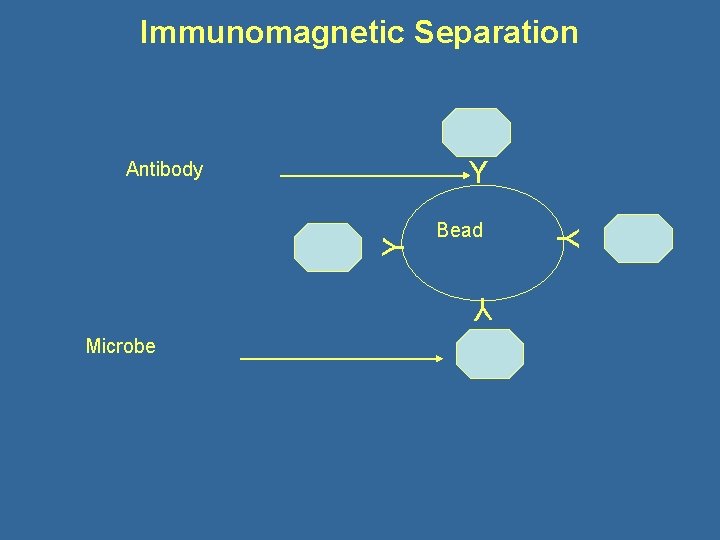
Immunomagnetic Separation Y Bead Y Microbe Y Y Antibody

Virus Capture Plus RT-PCR to Detect Infectious Viruses - The s. CAR System • The cell receptor gene for Coxsackieviruses and Adenoviruses has been cloned and expressed, producing a soluble protein receptor, s. CAR • Expressed, purified and bound s. CAR to solid phases to capture infectious Coxsackieviruses from environmental samples – The nucleic acid of the s. CAR-captured viruses is RT-PCR amplified for detection and quantitation
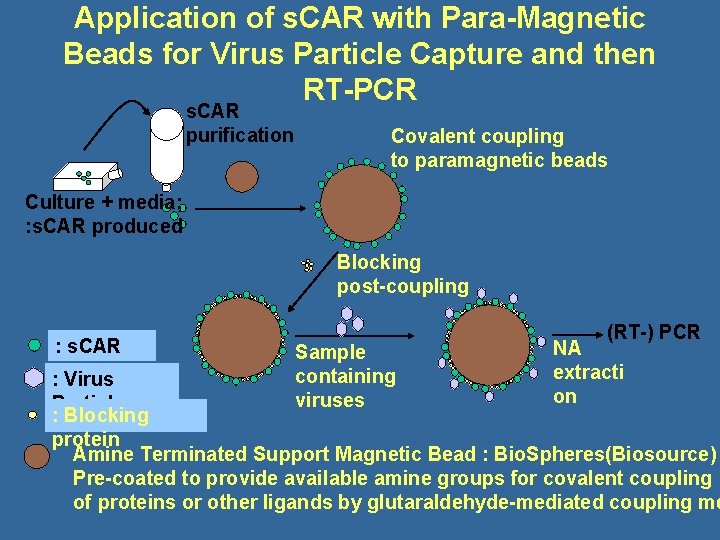
Application of s. CAR with Para-Magnetic Beads for Virus Particle Capture and then RT-PCR s. CAR purification Covalent coupling to paramagnetic beads Culture + media; : s. CAR produced Blocking post-coupling : s. CAR Sample containing viruses (RT-) PCR NA extracti on : Virus Particle : Blocking protein Amine Terminated Support Magnetic Bead : Bio. Spheres(Biosource) Pre-coated to provide available amine groups for covalent coupling of proteins or other ligands by glutaraldehyde-mediated coupling me
- Slides: 55