Micro Macro Unit 2 Nutrition Health Science 20
















- Slides: 59

Micro = ? Macro = ? Unit 2 - Nutrition Health Science 20 Outcome(s): HS 20 -NU 1 – Assess the importance of micro and macromolecules in maintaining Human health

Questions about Nutrition • What makes up our bodies? • What types of nutrients do we need? What are these composed of? • Do we need certain nutrients (ex. Fat, natural sugars)? • What nutrients are found in what foods? • What is a micronutrient?

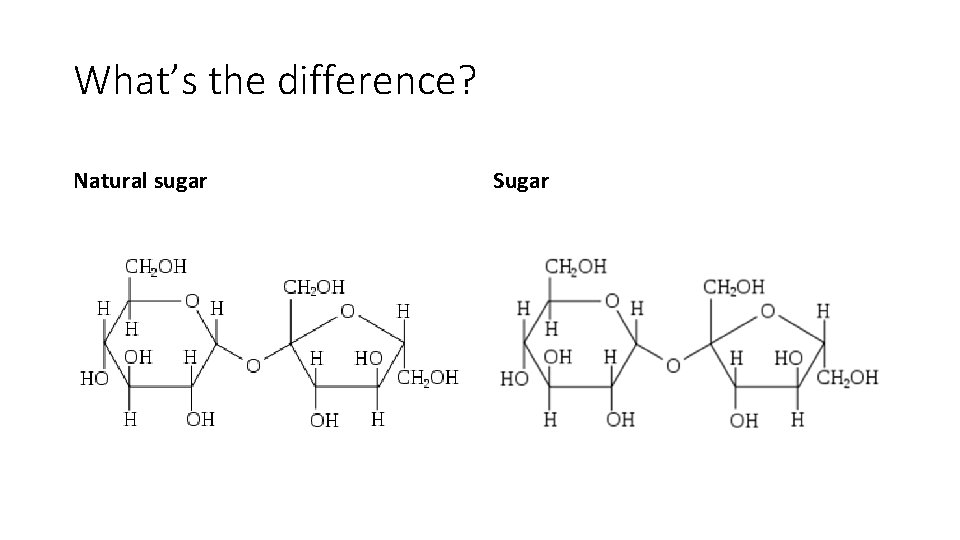
What’s the difference? Natural sugar Sugar

How does nutrition work? How does our body break down food? We need: - Water. - A mechanism to break down food. - A mechanism to put the component parts of food back together for specific functions.

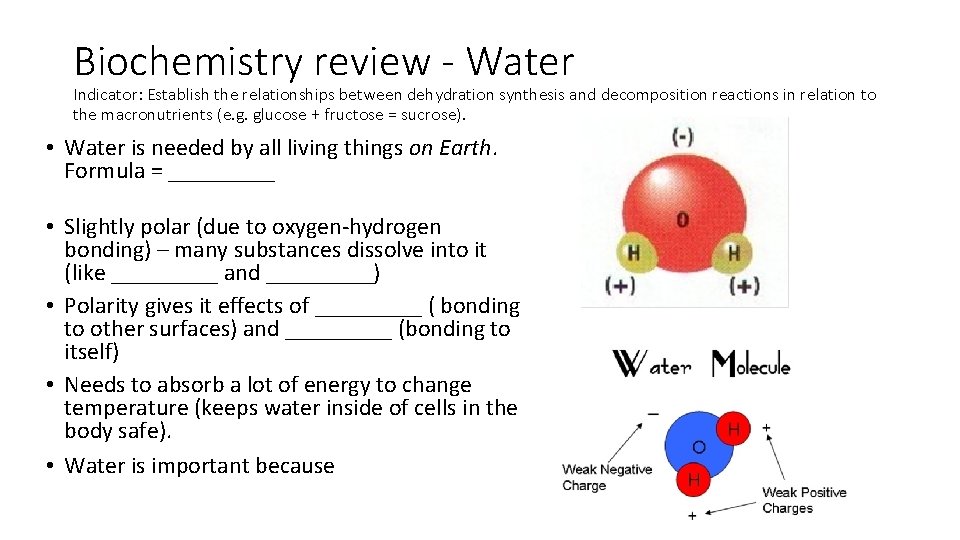
Biochemistry review - Water Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • Water is needed by all living things on Earth. Formula = _____ • Slightly polar (due to oxygen-hydrogen bonding) – many substances dissolve into it (like _____ and _____) • Polarity gives it effects of _____ ( bonding to other surfaces) and _____ (bonding to itself) • Needs to absorb a lot of energy to change temperature (keeps water inside of cells in the body safe). • Water is important because

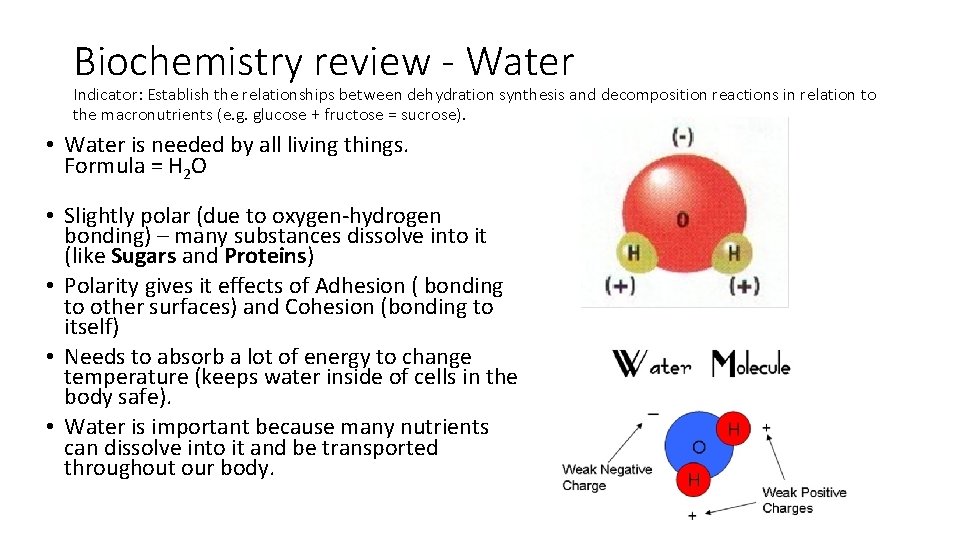
Biochemistry review - Water Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • Water is needed by all living things. Formula = H 2 O • Slightly polar (due to oxygen-hydrogen bonding) – many substances dissolve into it (like Sugars and Proteins) • Polarity gives it effects of Adhesion ( bonding to other surfaces) and Cohesion (bonding to itself) • Needs to absorb a lot of energy to change temperature (keeps water inside of cells in the body safe). • Water is important because many nutrients can dissolve into it and be transported throughout our body.

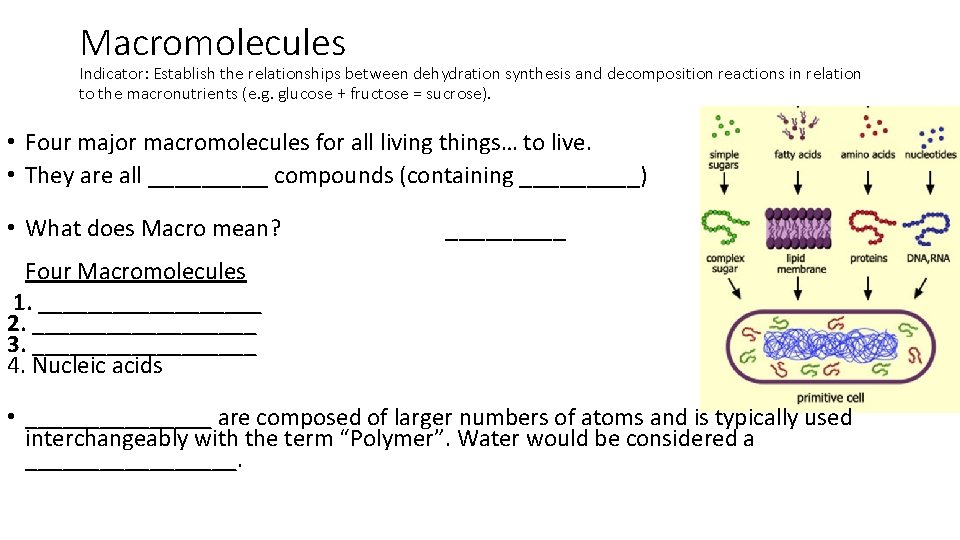
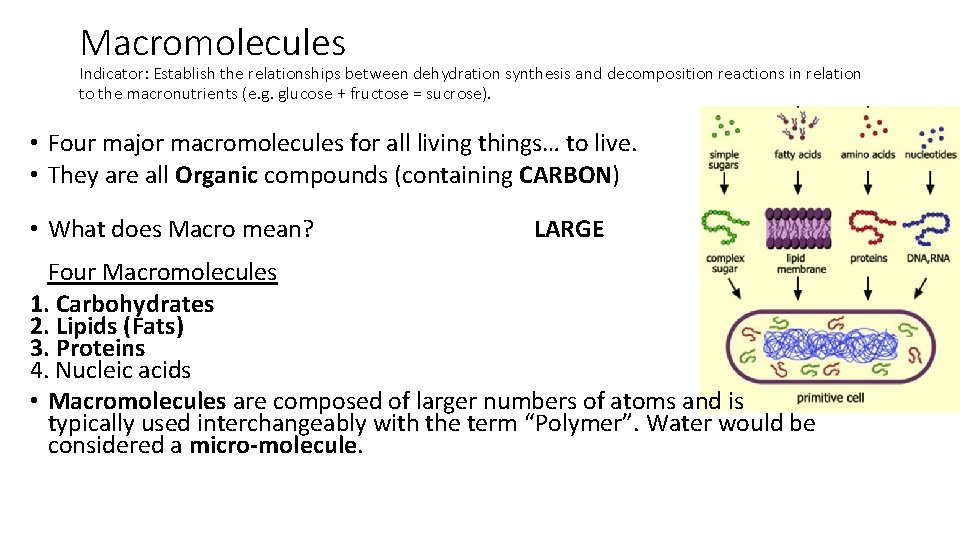
Macromolecules Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • Four major macromolecules for all living things… to live. • They are all _____ compounds (containing _____) • What does Macro mean? _____ Four Macromolecules 1. _________ 2. _________ 3. _________ 4. Nucleic acids • ________ are composed of larger numbers of atoms and is typically used interchangeably with the term “Polymer”. Water would be considered a _________.

Macromolecules Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • Four major macromolecules for all living things… to live. • They are all Organic compounds (containing CARBON) • What does Macro mean? LARGE Four Macromolecules 1. Carbohydrates 2. Lipids (Fats) 3. Proteins 4. Nucleic acids • Macromolecules are composed of larger numbers of atoms and is typically used interchangeably with the term “Polymer”. Water would be considered a micro-molecule.

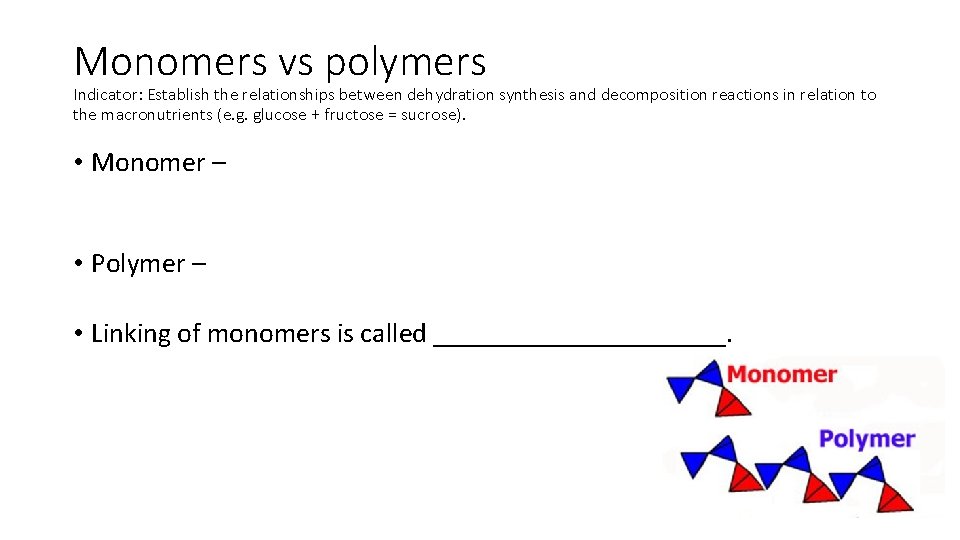
Monomers vs polymers Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • Monomer – • Polymer – • Linking of monomers is called ___________.

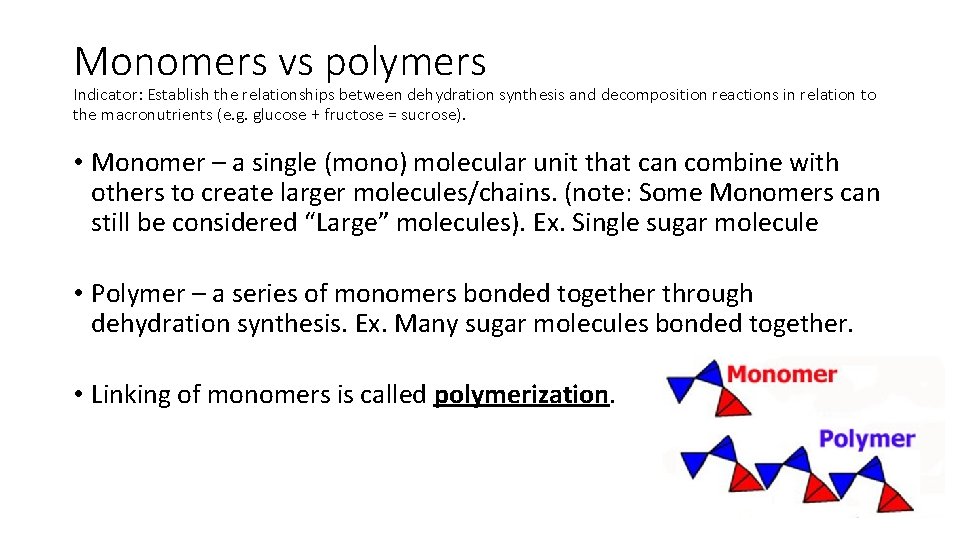
Monomers vs polymers Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • Monomer – a single (mono) molecular unit that can combine with others to create larger molecules/chains. (note: Some Monomers can still be considered “Large” molecules). Ex. Single sugar molecule • Polymer – a series of monomers bonded together through dehydration synthesis. Ex. Many sugar molecules bonded together. • Linking of monomers is called polymerization.

Connecting it together • We use water (and enzymes) to break down (decompose) nutrients (macromolecules in the form of polymers into monomers). • Systems in our body (circulatory in association with the digestive) transports these molecular components to places that its needed in the body – where they may be synthesized to make new polymers (proteins, sugars, or fats). • How exactly does this happen though?

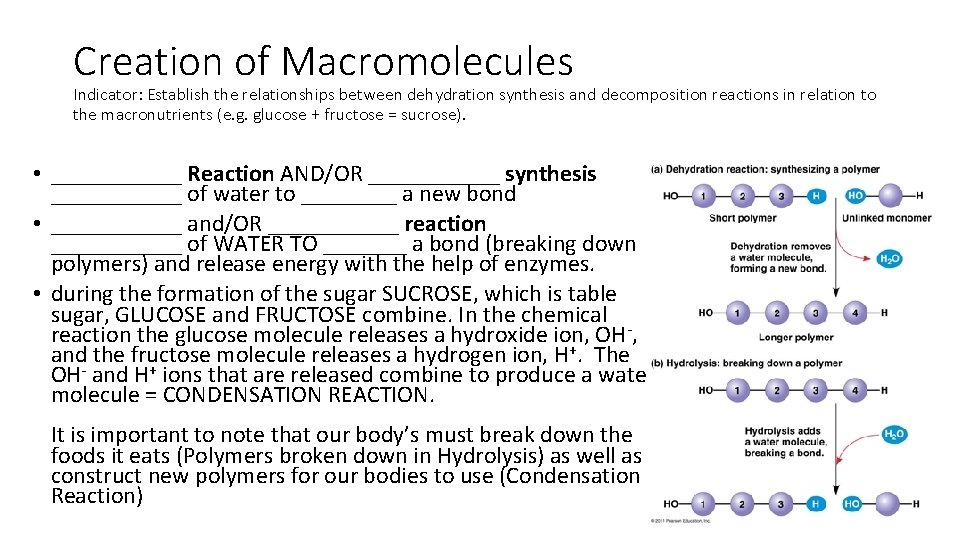
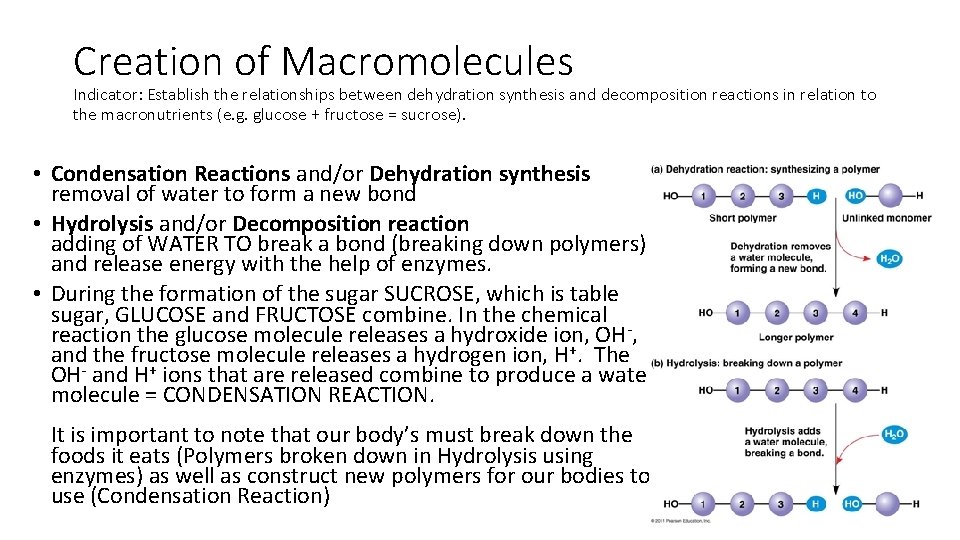
Creation of Macromolecules Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • ______ Reaction AND/OR ______ synthesis ______ of water to ____ a new bond • ______ and/OR ______ reaction ______ of WATER TO _______ a bond (breaking down polymers) and release energy with the help of enzymes. • during the formation of the sugar SUCROSE, which is table sugar, GLUCOSE and FRUCTOSE combine. In the chemical reaction the glucose molecule releases a hydroxide ion, OH-, and the fructose molecule releases a hydrogen ion, H+. The OH- and H+ ions that are released combine to produce a water molecule = CONDENSATION REACTION. It is important to note that our body’s must break down the foods it eats (Polymers broken down in Hydrolysis) as well as construct new polymers for our bodies to use (Condensation Reaction)

Creation of Macromolecules Indicator: Establish the relationships between dehydration synthesis and decomposition reactions in relation to the macronutrients (e. g. glucose + fructose = sucrose). • Condensation Reactions and/or Dehydration synthesis removal of water to form a new bond • Hydrolysis and/or Decomposition reaction adding of WATER TO break a bond (breaking down polymers) and release energy with the help of enzymes. • During the formation of the sugar SUCROSE, which is table sugar, GLUCOSE and FRUCTOSE combine. In the chemical reaction the glucose molecule releases a hydroxide ion, OH-, and the fructose molecule releases a hydrogen ion, H+. The OH- and H+ ions that are released combine to produce a water molecule = CONDENSATION REACTION. It is important to note that our body’s must break down the foods it eats (Polymers broken down in Hydrolysis using enzymes) as well as construct new polymers for our bodies to use (Condensation Reaction)

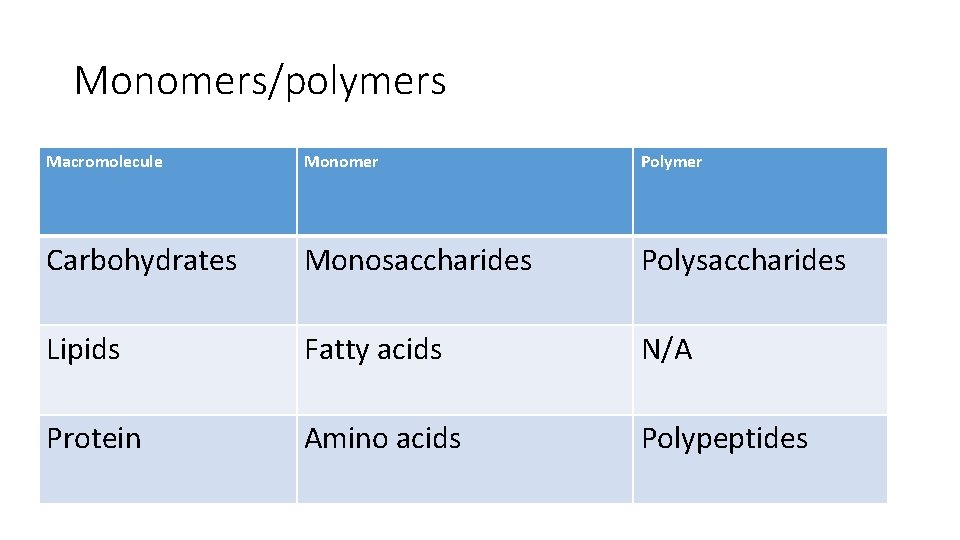
Monomers/polymers Macromolecule Monomer Polymer Carbohydrates Monosaccharides Polysaccharides Lipids Fatty acids N/A Protein Amino acids Polypeptides

Carbohydrates Indicator: Examine the role of carbohydrates (monosaccharides, disaccharides, and polysaccharides) as being the main source of short term energy. • ______________ and storage for humans – how is it used? _____ (A hormone that binds with and allows sugar to go into cells). Once inside, Sugar’s bonds are easy to break (Energy is stored in the bonds) three forms of carbs are mono-, Di-, and Poly-saccharides • Monomer = _______ (Glucose, fructose, galactose) _____ - all of these have the same formula, different structure

Carbohydrates Indicator: Examine the role of carbohydrates (monosaccharides, disaccharides, and polysaccharides) as being the main source of short term energy. • Primary short-term energy Source and storage for humans – how is it used? Insulin (A hormone that binds with and allows sugar to go into cells). Once inside, Sugar’s bonds are easy to break (Energy is stored in the bonds) three forms of carbs are mono-, Di-, and Poly-saccharides • Monomer = Monosaccharide (Glucose, fructose, galactose) Isomers - all of these have the same formula, different structure

Carbohydrates Indicator: Examine the role of carbohydrates (monosaccharides, disaccharides, and polysaccharides) as being the main source of short term energy. • Chemical formulas are multiples of CH 2 O, composed of the elements • Two monomers = ________ (sucrose – table sugar or lactose) • Several Monomers = Polymer = ________ – starch (bread, pasta), cellulose (plant cell walls), glycogen (quick energy – hundreds of glucose bonded together)

Carbohydrates Indicator: Examine the role of carbohydrates (monosaccharides, disaccharides, and polysaccharides) as being the main source of short term energy. • Chemical formulas are multiples of CH 2 O, composed of the elements Carbon, hydrogen, and Oxygen • Two monomers = Disaccharides (sucrose – table sugar or lactose) • Several Monomers = Polymer = polysaccharides – starch (bread, pasta), cellulose (plant cell walls), glycogen (quick energy – hundreds of glucose bonded together)

Lipids – Cell membranes and hormone synthesis Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. • Lipids – composed of their monomer ______ they don’t mix with water – hydrophobic – large and nonpolar – this allows it to store/transport nonpolar nutrients (Certain vitamins)! • Lipid molecules have a HIGHER ratio of _____ and ______ atoms to ______ atoms than carbohydrates have. More C, H than O

Lipids – Cell membranes and hormone synthesis Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. • Lipids – composed of their monomer fatty acids they don’t mix with water – hydrophobic – large and nonpolar – this allows it to store/transport nonpolar nutrients! • Lipid molecules have a HIGHER ratio of carbon and hydrogen atoms to oxygen atoms than carbohydrates have. More C, H than O

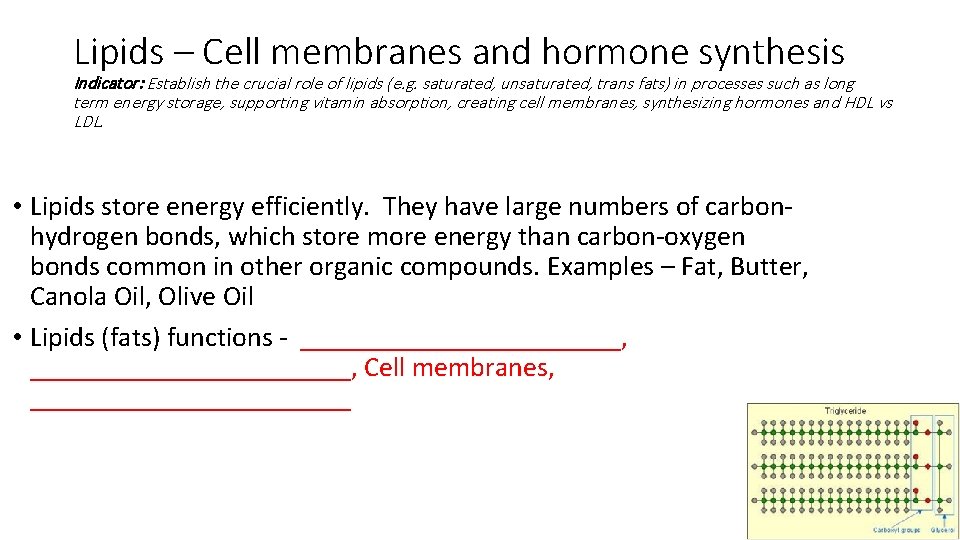
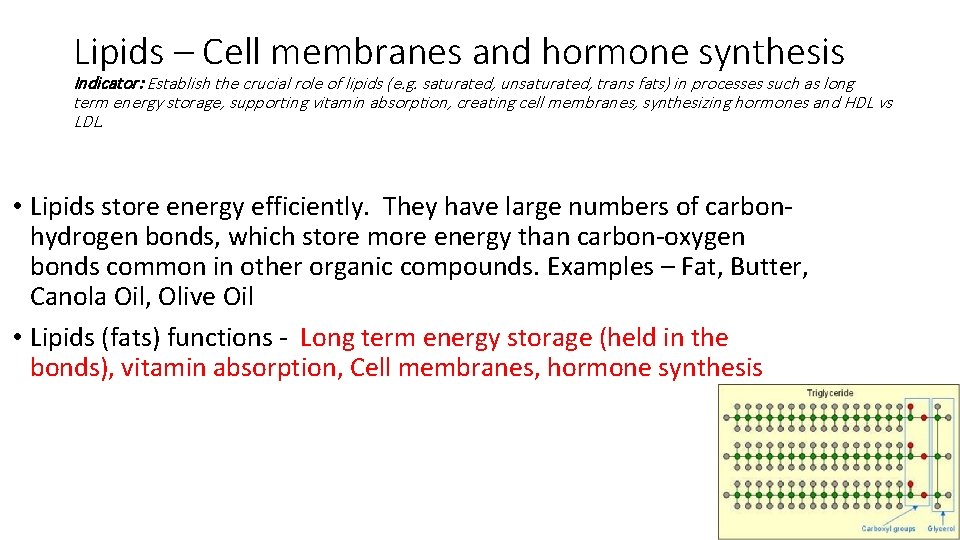
Lipids – Cell membranes and hormone synthesis Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. • Lipids store energy efficiently. They have large numbers of carbonhydrogen bonds, which store more energy than carbon-oxygen bonds common in other organic compounds. Examples – Fat, Butter, Canola Oil, Olive Oil • Lipids (fats) functions - _______________________, Cell membranes, ____________

Lipids – Cell membranes and hormone synthesis Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. • Lipids store energy efficiently. They have large numbers of carbonhydrogen bonds, which store more energy than carbon-oxygen bonds common in other organic compounds. Examples – Fat, Butter, Canola Oil, Olive Oil • Lipids (fats) functions - Long term energy storage (held in the bonds), vitamin absorption, Cell membranes, hormone synthesis

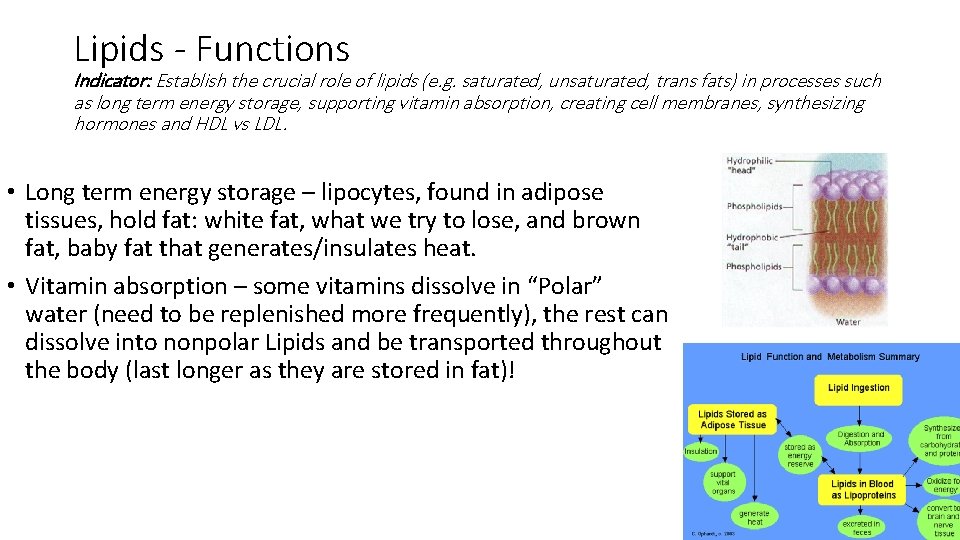
Lipids - Functions Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. • Long term energy storage – lipocytes, found in adipose tissues, hold fat: white fat, what we try to lose, and brown fat, baby fat that generates/insulates heat. • Vitamin absorption – some vitamins dissolve in “Polar” water (need to be replenished more frequently), the rest can dissolve into nonpolar Lipids and be transported throughout the body (last longer as they are stored in fat)!

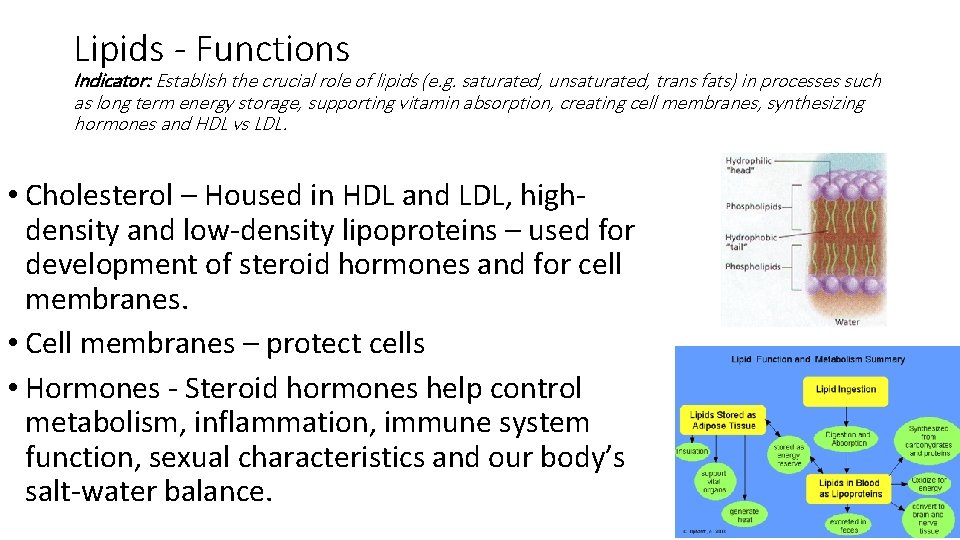
Lipids - Functions Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. • Cholesterol – Housed in HDL and LDL, highdensity and low-density lipoproteins – used for development of steroid hormones and for cell membranes. • Cell membranes – protect cells • Hormones - Steroid hormones help control metabolism, inflammation, immune system function, sexual characteristics and our body’s salt-water balance.

Types of Lipids Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. Types of lipids • __________ – have no double bonds – ____ at room temperature • ____________ – have double bonds – ____ at room temperature • ____________– a type of synthetic unsaturated fatty acid – raising LDL, lowering HDL • Fatty acid-based lipids – triglycerides, phospholipids, wax • Steroids - rings – cholesterol, testosterone

Lipids Indicator: Establish the crucial role of lipids (e. g. saturated, unsaturated, trans fats) in processes such as long term energy storage, supporting vitamin absorption, creating cell membranes, synthesizing hormones and HDL vs LDL. Types of lipids • Saturated – have no double bonds – solid at room temperature • Unsaturated – have double bonds – liquid at room temperature • Trans Fats – a type of synthetic unsaturated fatty acid – raising LDL, lowering HDL • Fatty acid-based lipids – triglycerides, phospholipids, wax • Steroids - rings – cholesterol, testosterone

What is cholesterol for? • What has LDL in it? What does it do? • What has HDL in it? What does it do? • Which cholesterol is “Bad” cholesterol?

What is cholesterol for? • What has LDL in it? What does it do? Fatty Meats, Milk, Cheese, Butter, Yogurt Carries cholesterol and can leave it in your arteries https: //www. youtube. com/watch ? v=5 w. Gai. Li. ADBs Cholesterol Reducing Technology • What has HDL in it? What does it do? Composed of several protein – found in fish, Beans/Legumes, nuts, Flax, CHIA. Lowers/gets rid of LDL build-up in arteries. • Which cholesterol is “Bad” cholesterol? Low-density lipoproteins (LDL)

Quiz Questions What are the four macromolecules? • What are the elements that make up Carbohydrates? • What is the function of carbohydrates? • What are the monomers of carbohydrates? Provide the term and an example • What are carbohydrates found in (foods)? • What are the elements that make up Lipids? • What is the function of Lipids (List 2)? • What are the monomers of Lipids?

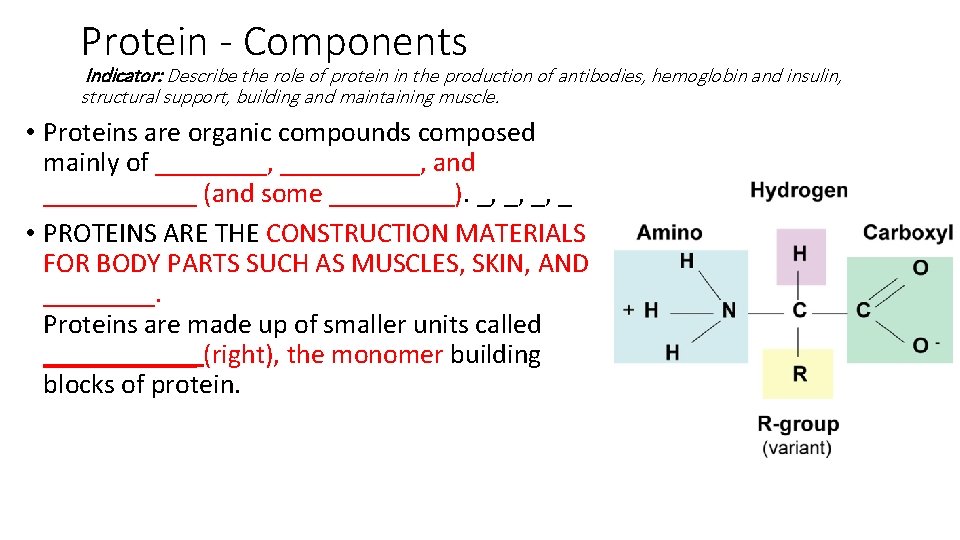
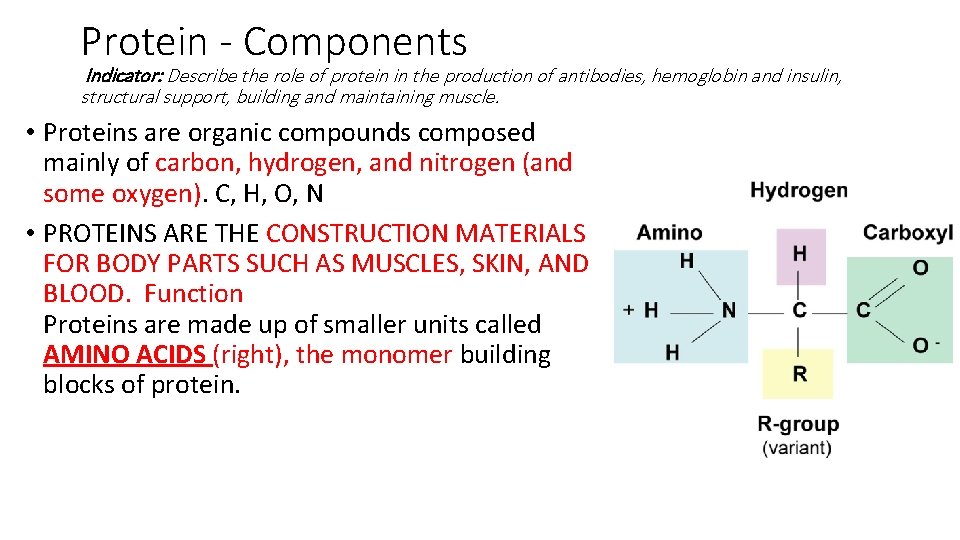
Protein - Components Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. • Proteins are organic compounds composed mainly of ____, _____, and ______ (and some _____). _, _, _, _ • PROTEINS ARE THE CONSTRUCTION MATERIALS FOR BODY PARTS SUCH AS MUSCLES, SKIN, AND ____. Proteins are made up of smaller units called ______ (right), the monomer building blocks of protein.

Protein - Components Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. • Proteins are organic compounds composed mainly of carbon, hydrogen, and nitrogen (and some oxygen). C, H, O, N • PROTEINS ARE THE CONSTRUCTION MATERIALS FOR BODY PARTS SUCH AS MUSCLES, SKIN, AND BLOOD. Function Proteins are made up of smaller units called AMINO ACIDS (right), the monomer building blocks of protein.

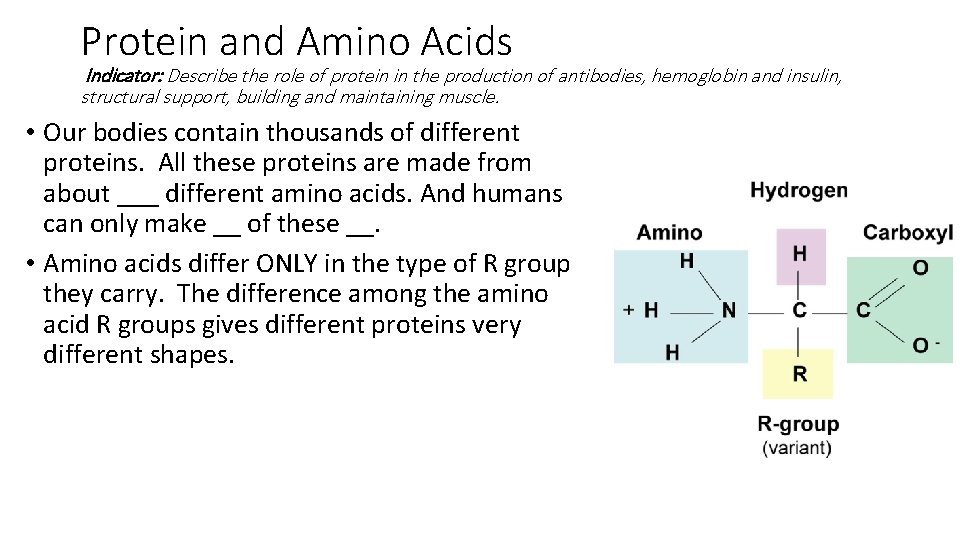
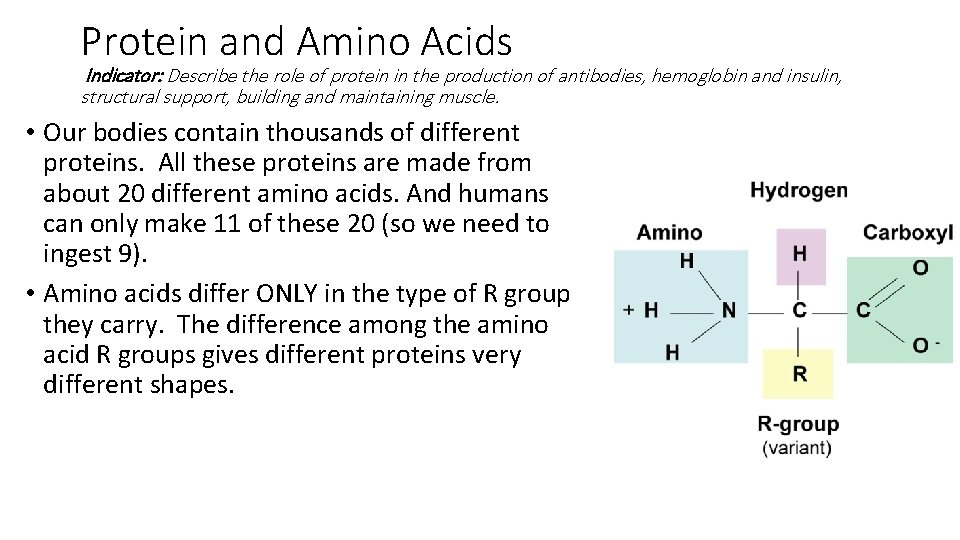
Protein and Amino Acids Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. • Our bodies contain thousands of different proteins. All these proteins are made from about ___ different amino acids. And humans can only make __ of these __. • Amino acids differ ONLY in the type of R group they carry. The difference among the amino acid R groups gives different proteins very different shapes.

Protein and Amino Acids Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. • Our bodies contain thousands of different proteins. All these proteins are made from about 20 different amino acids. And humans can only make 11 of these 20 (so we need to ingest 9). • Amino acids differ ONLY in the type of R group they carry. The difference among the amino acid R groups gives different proteins very different shapes.

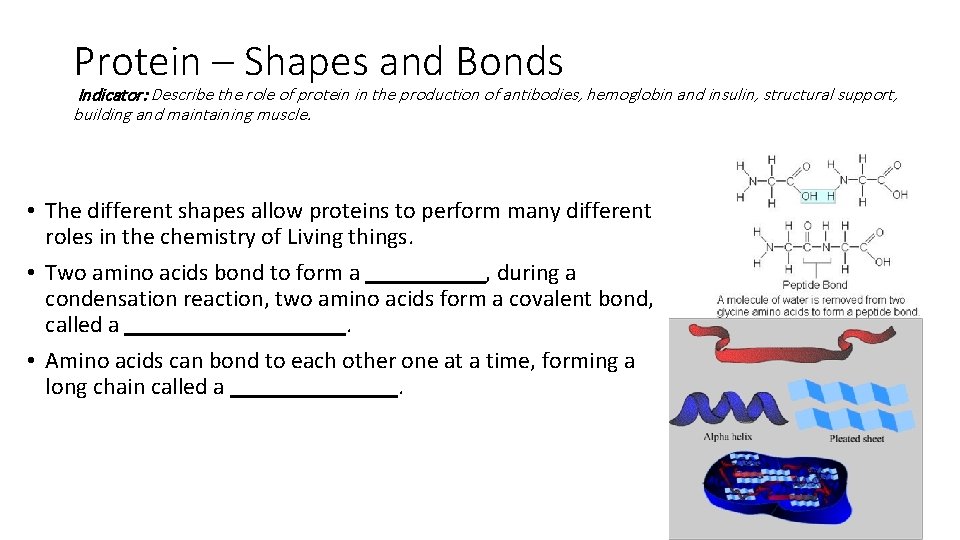
Protein – Shapes and Bonds Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. • The different shapes allow proteins to perform many different roles in the chemistry of Living things. • Two amino acids bond to form a _____, during a condensation reaction, two amino acids form a covalent bond, called a _____. • Amino acids can bond to each other one at a time, forming a long chain called a _______.

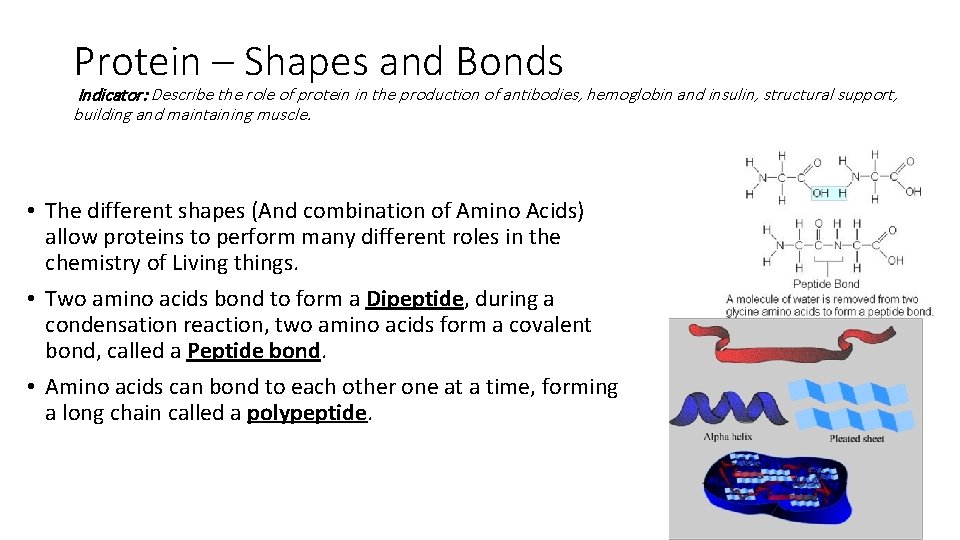
Protein – Shapes and Bonds Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. • The different shapes (And combination of Amino Acids) allow proteins to perform many different roles in the chemistry of Living things. • Two amino acids bond to form a Dipeptide, during a condensation reaction, two amino acids form a covalent bond, called a Peptide bond. • Amino acids can bond to each other one at a time, forming a long chain called a polypeptide.

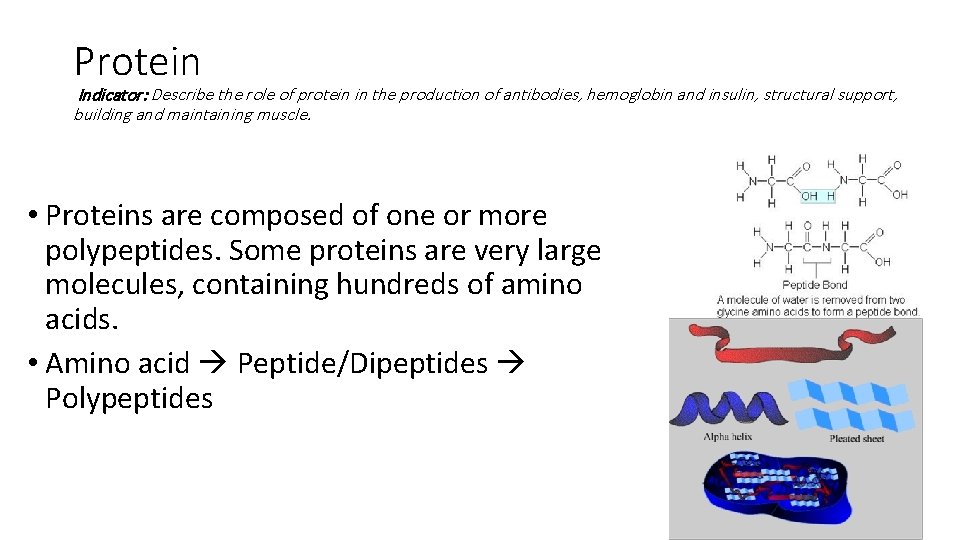
Protein Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. • Proteins are composed of one or more polypeptides. Some proteins are very large molecules, containing hundreds of amino acids. • Amino acid Peptide/Dipeptides Polypeptides

Protein Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. Functions • Antibodies – globular proteins with sugars attached to amino acid chains – they identify foreign macromolecules and bind to them, eventually destroying them • Hemoglobin – a protein in red blood cells that hold oxygen • Insulin – a protein regulates metabolism (energy uptake)

Protein Indicator: Describe the role of protein in the production of antibodies, hemoglobin and insulin, structural support, building and maintaining muscle. Functions • Structural support – proteins make up most structures in the body • Building and maintaining muscle – muscle tissue is built from amino acids, when muscles undergo stress/Damage, they need to be repaired.

Crash Course! • I like John more than hank… but this is a good overview of what we have discussed! https: //www. youtube. com/watch? v=H 8 WJ 2 KENl. K 0 • Remember: In biology, there’s almost always information available online for our topics. • Write down three points that stood out from the video to share.

Lab – building macromolecules • You may work in partners at your own pace or follow along with instructor

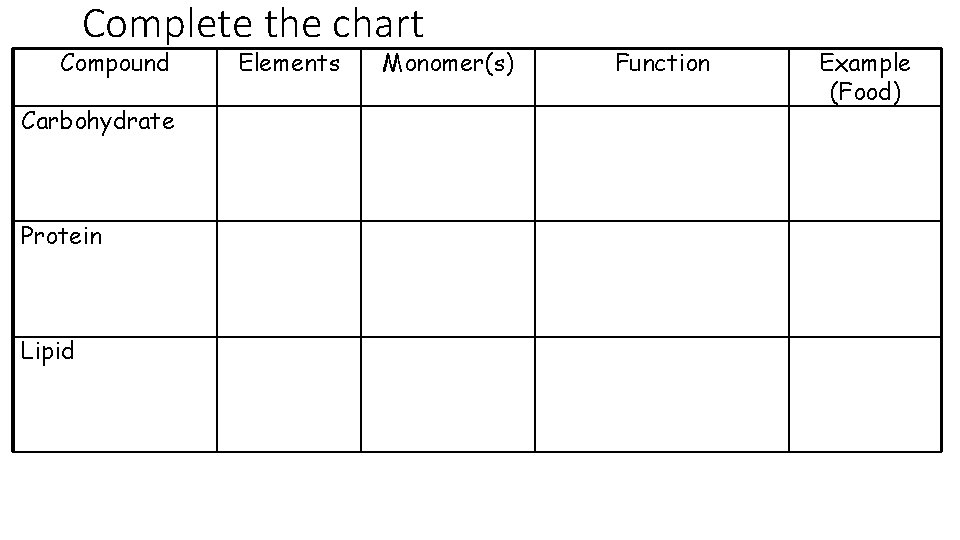
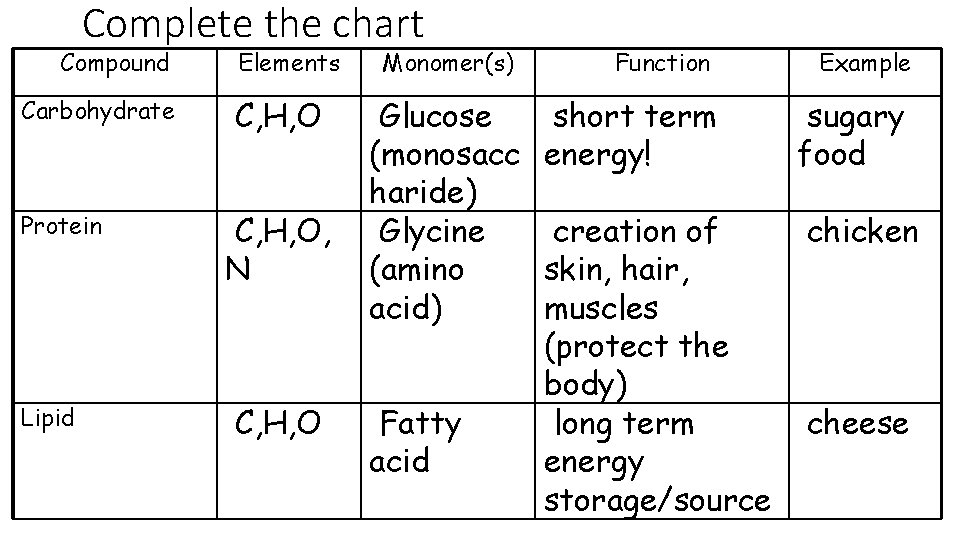
Complete the chart Compound Carbohydrate Protein Lipid Elements Monomer(s) Function Example (Food)

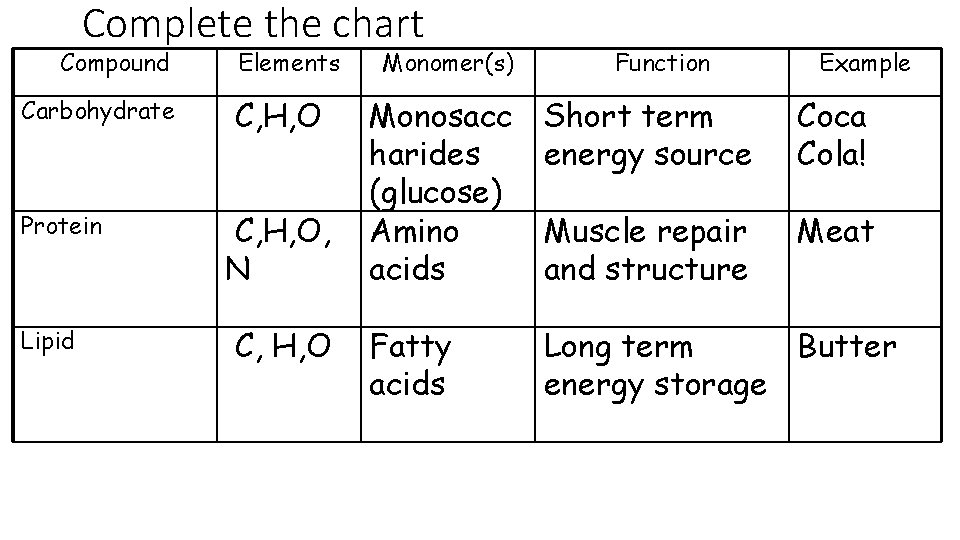
Complete the chart Compound Carbohydrate Protein Lipid Elements C, H, O, N C, H, O Monomer(s) Function Example Monosacc harides (glucose) Amino acids Short term energy source Coca Cola! Muscle repair and structure Meat Fatty acids Long term Butter energy storage

Complete the chart Compound Carbohydrate Protein Lipid Elements C, H, O, N C, H, O Monomer(s) Function Example Glucose short term sugary (monosacc energy! food haride) Glycine creation of chicken (amino skin, hair, acid) muscles (protect the body) Fatty long term cheese acid energy storage/source

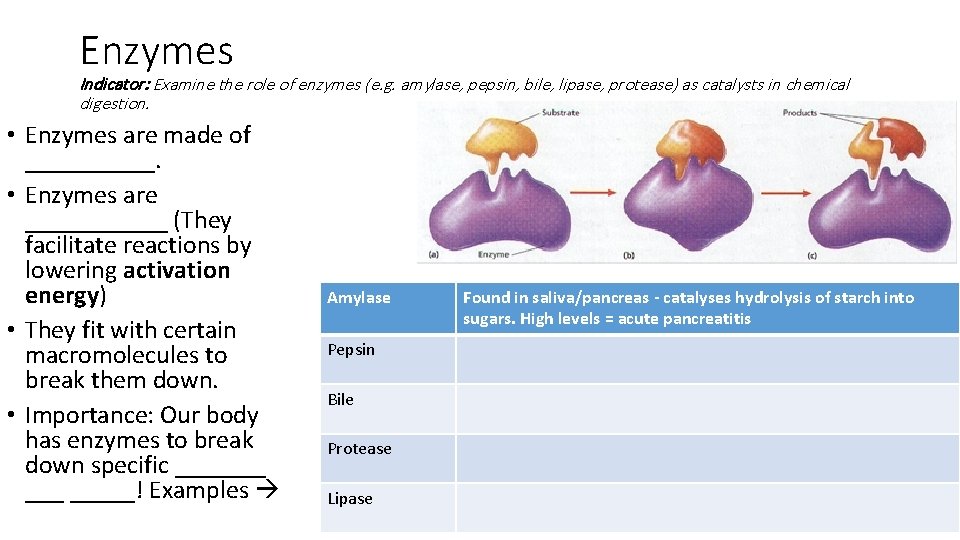
Enzymes Indicator: Examine the role of enzymes (e. g. amylase, pepsin, bile, lipase, protease) as catalysts in chemical digestion. • Enzymes are made of _____. • Enzymes are ______ (They facilitate reactions by lowering activation energy) • They fit with certain macromolecules to break them down. • Importance: Our body has enzymes to break down specific _______! Examples Amylase Pepsin Bile Protease Lipase Found in saliva/pancreas - catalyses hydrolysis of starch into sugars. High levels = acute pancreatitis

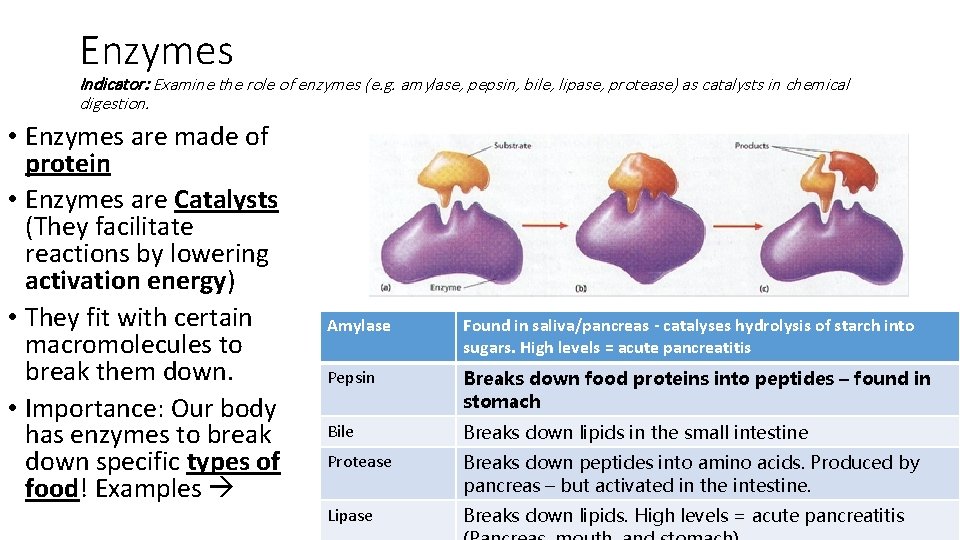
Enzymes Indicator: Examine the role of enzymes (e. g. amylase, pepsin, bile, lipase, protease) as catalysts in chemical digestion. • Enzymes are made of protein • Enzymes are Catalysts (They facilitate reactions by lowering activation energy) • They fit with certain macromolecules to break them down. • Importance: Our body has enzymes to break down specific types of food! Examples Amylase Found in saliva/pancreas - catalyses hydrolysis of starch into sugars. High levels = acute pancreatitis Pepsin Breaks down food proteins into peptides – found in stomach Bile Breaks down lipids in the small intestine Protease Breaks down peptides into amino acids. Produced by pancreas – but activated in the intestine. Lipase Breaks down lipids. High levels = acute pancreatitis

Quiz • Matching Enzymes with where they are found and their function. (5) • Two functions of proteins (2) • What elements make up proteins? What is their monomer? (2) • What do enzymes do? What are they made of? (2)

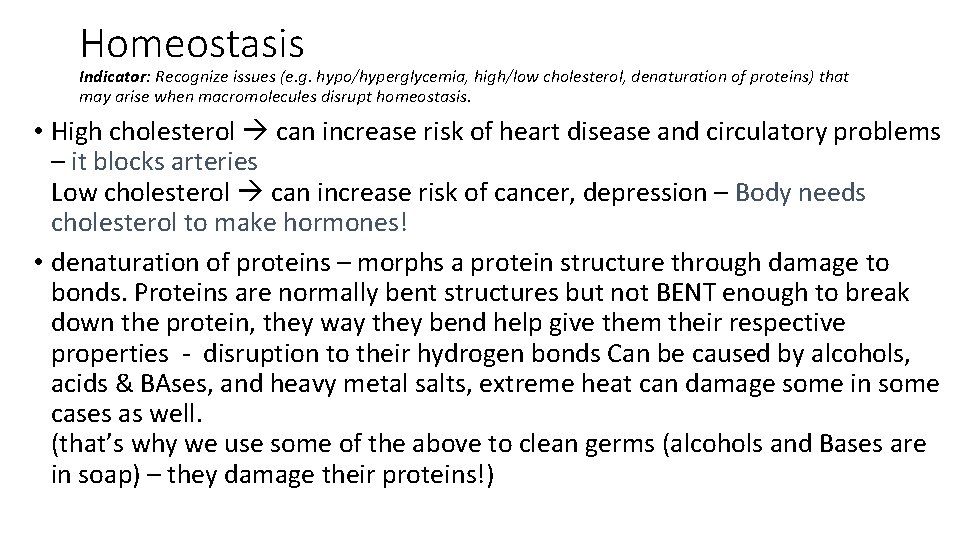
Homeostasis Indicator: Recognize issues (e. g. hypo/hyperglycemia, high/low cholesterol, denaturation of proteins) that may arise when macromolecules disrupt homeostasis. • Homeostasis = Example – • How do macromolecules disrupt homeostasis? • Glycemia = levels of glucose in the blood (hypo – ______, hyper – _____) _____ dizziness, confusion, weakness – short on energy, body responds this way _____ increased thirst and urination – Lots of energy, body needs to replenish excessive water use WHY Would these symptoms occur?

Homeostasis Indicator: Recognize issues (e. g. hypo/hyperglycemia, high/low cholesterol, denaturation of proteins) that may arise when macromolecules disrupt homeostasis. • Homeostasis = balance/Stability Our bodies Need to maintain balance! Example – Working out… your body may start to sweat to cool off. • How do macromolecules disrupt homeostasis? Too much or not enough for the body to adapt to! That’s why you need a balanced diet! • Glycemia = levels of glucose in the blood (hypo – low, hyper – high) hypo dizziness, confusion, weakness – short on energy, body responds this way hyper increased thirst and urination – Lots of energy, body needs to replenish excessive water use WHY Would these symptoms occur?

Homeostasis Indicator: Recognize issues (e. g. hypo/hyperglycemia, high/low cholesterol, denaturation of proteins) that may arise when macromolecules disrupt homeostasis. • High cholesterol can increase risk of heart disease and circulatory problems – it blocks arteries Low cholesterol can increase risk of cancer, depression – Body needs cholesterol to make hormones! • denaturation of proteins – morphs a protein structure through damage to bonds. Proteins are normally bent structures but not BENT enough to break down the protein, they way they bend help give them their respective properties - disruption to their hydrogen bonds Can be caused by alcohols, acids & BAses, and heavy metal salts, extreme heat can damage some in some cases as well. (that’s why we use some of the above to clean germs (alcohols and Bases are in soap) – they damage their proteins!)

People making nutrition contributions Investigate the contributions of people (e. g. , Justus von Liebig, Antoine-Laurent Lavoisier, Claude Bernard, and Emil Fischer) in advancing scientific understanding of nutrition. (STSE) Use your phone! What do you think these individuals contributed to health science and why is their discovery important? • Justus von liebig – • Antoine-Laurent de Lavoisier – • Claude Bernard – • Emil fischer –

People making nutrition contributions Investigate the contributions of people (e. g. , Justus von Liebig, Antoine-Laurent Lavoisier, Claude Bernard, and Emil Fischer) in advancing scientific understanding of nutrition. (STSE) Use your phone! What do you think these individuals contributed to health science and why is their discovery important? • Justus von liebig – German chemist – Biological chemistry – PEOPLE GOTTA EAT! • Antoine-Laurent de Lavoisier – recognized and named oxygen and hydrogen • Claude Bernard – Came up with the term Homeostasis • Emil fischer – drawing of atoms!

Contributions – expect on quiz • Justus von Liebig – father of organic chemistry and fertilizer – Law of the minimum – plants grow only as much as the scarcest resource. Connecting to us and nutrition – we can only grow as much as we ingest, if we’re lacking a particular nutrient – we would have deficiencies • Antoine-Laurent De Lavoisier – recognized and named oxygen and hydrogen – and that while matter may change shape, mass stays the same. Oxygen and hydrogen are found in all macromolecules! • Claude Bernard – blind experiments and homeostasis – Important: Our bodies constantly try to achieve balance • Emil Fischer – way of drawing carbon molecules – this allowed us to observe how macromolecules may behave given their structure Importance: While not all directly related to nutrition. Their discoveries helped inform our understanding of health Science – which in turn helped with

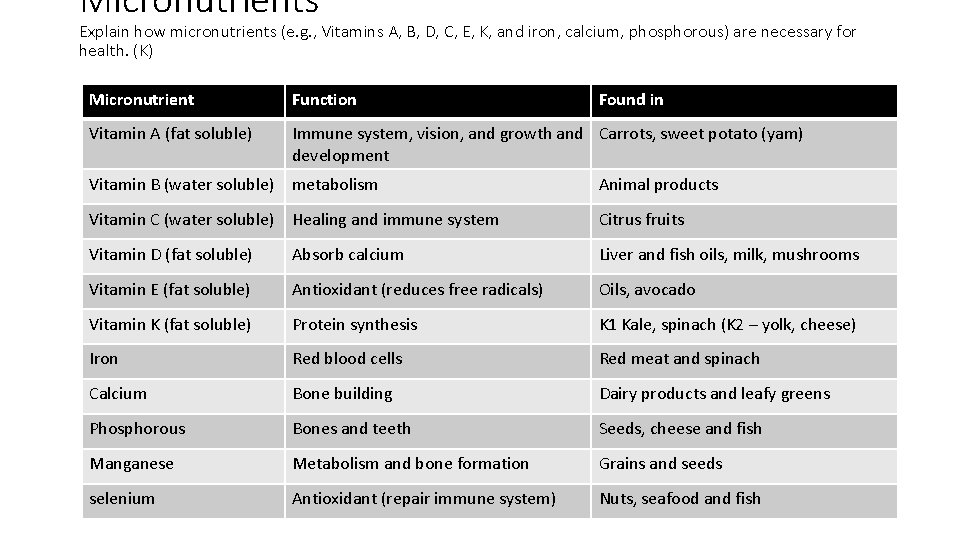
Micronutrients Explain how micronutrients (e. g. , Vitamins A, B, D, C, E, K, and iron, calcium, phosphorous) are necessary for health. (K) Micronutrient Vitamin A (fat soluble) Vitamin B (water soluble) Vitamin C (water soluble) Vitamin D (fat soluble) Vitamin E (fat soluble) Vitamin K (fat soluble) Iron Calcium Phosphorous Manganese selenium Function Found in

Micronutrients Explain how micronutrients (e. g. , Vitamins A, B, D, C, E, K, and iron, calcium, phosphorous) are necessary for health. (K) Micronutrient Function Found in Vitamin A (fat soluble) Immune system, vision, and growth and Carrots, sweet potato (yam) development Vitamin B (water soluble) metabolism Animal products Vitamin C (water soluble) Healing and immune system Citrus fruits Vitamin D (fat soluble) Absorb calcium Liver and fish oils, milk, mushrooms Vitamin E (fat soluble) Antioxidant (reduces free radicals) Oils, avocado Vitamin K (fat soluble) Protein synthesis K 1 Kale, spinach (K 2 – yolk, cheese) Iron Red blood cells Red meat and spinach Calcium Bone building Dairy products and leafy greens Phosphorous Bones and teeth Seeds, cheese and fish Manganese Metabolism and bone formation Grains and seeds selenium Antioxidant (repair immune system) Nuts, seafood and fish

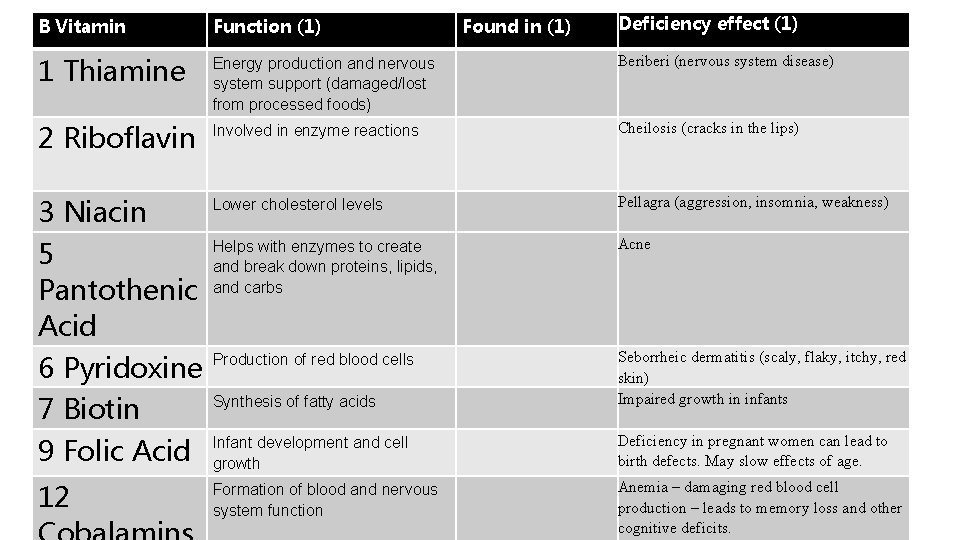
B Vitamins • https: //en. wikipedia. org/wiki/B_vitamins B Vitamin 1 2 3 5 6 7 9 12 Function Found in

Found in (1) Deficiency effect (1) B Vitamin Function (1) 1 Thiamine Energy production and nervous system support (damaged/lost from processed foods) Beriberi (nervous system disease) 2 Riboflavin Involved in enzyme reactions Cheilosis (cracks in the lips) 3 Niacin 5 Pantothenic Acid 6 Pyridoxine 7 Biotin 9 Folic Acid Lower cholesterol levels Pellagra (aggression, insomnia, weakness) Helps with enzymes to create and break down proteins, lipids, and carbs Acne Production of red blood cells Synthesis of fatty acids Seborrheic dermatitis (scaly, flaky, itchy, red skin) Impaired growth in infants Infant development and cell growth Deficiency in pregnant women can lead to birth defects. May slow effects of age. 12 Formation of blood and nervous system function Anemia – damaging red blood cell production – leads to memory loss and other cognitive deficits.

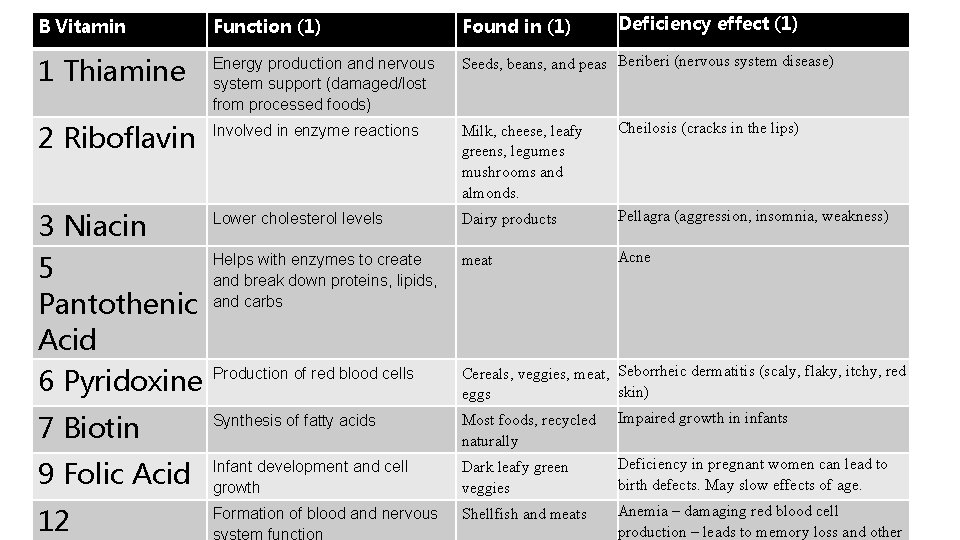
Deficiency effect (1) B Vitamin Function (1) Found in (1) 1 Thiamine Energy production and nervous system support (damaged/lost from processed foods) Seeds, beans, and peas Beriberi (nervous system disease) 2 Riboflavin Involved in enzyme reactions Milk, cheese, leafy greens, legumes mushrooms and almonds. Cheilosis (cracks in the lips) 3 Niacin 5 Pantothenic Acid 6 Pyridoxine Lower cholesterol levels Dairy products Pellagra (aggression, insomnia, weakness) Helps with enzymes to create and break down proteins, lipids, and carbs meat Acne Production of red blood cells 7 Biotin Synthesis of fatty acids 9 Folic Acid Infant development and cell growth 12 Formation of blood and nervous system function Cereals, veggies, meat, Seborrheic dermatitis (scaly, flaky, itchy, red skin) eggs Most foods, recycled Impaired growth in infants naturally Deficiency in pregnant women can lead to Dark leafy green birth defects. May slow effects of age. veggies Anemia – damaging red blood cell Shellfish and meats production – leads to memory loss and other

• 2 Riboflavin • Involved in enzyme reactions • Milk cheese, leafy greens, legumes mushrooms and almonds. • Cheilosis (cracks in the lips)

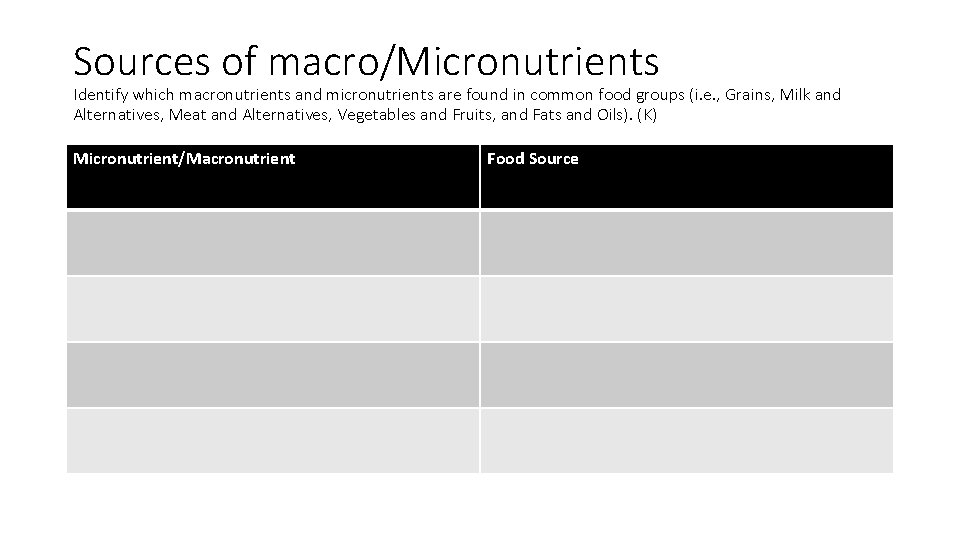
Sources of macro/Micronutrients Identify which macronutrients and micronutrients are found in common food groups (i. e. , Grains, Milk and Alternatives, Meat and Alternatives, Vegetables and Fruits, and Fats and Oils). (K) Micronutrient/Macronutrient Food Source