MICRO AND MACRODISPERSION OF SILICAFILLED TIRE TREAD COMPOUNDS







- Slides: 26

MICRO- AND MACRO-DISPERSION OF SILICA-FILLED TIRE TREAD COMPOUNDS Dorien Boiten, Wisut Kaewsakul, Wilma Dierkes, Jacques Noordermeer and Anke Blume Elastomer Technology and Engineering (ETE), Department of Mechanics of Solids and Systems (MS 3), Faculty of Engineering Technology, University of Twente, The Netherlands Presented at Tire Technology Conference, March 5 -7, 2019 Hanover, Germany

OUTLINE Ø Background ü Reinforcing concept ü Dispersion of filler Ø Research objectives ü Motivation ü Correlation between macro- and micro-dispersion Ø Experimental Ø Results Ø Conclusions 2

1. BACKGROUND

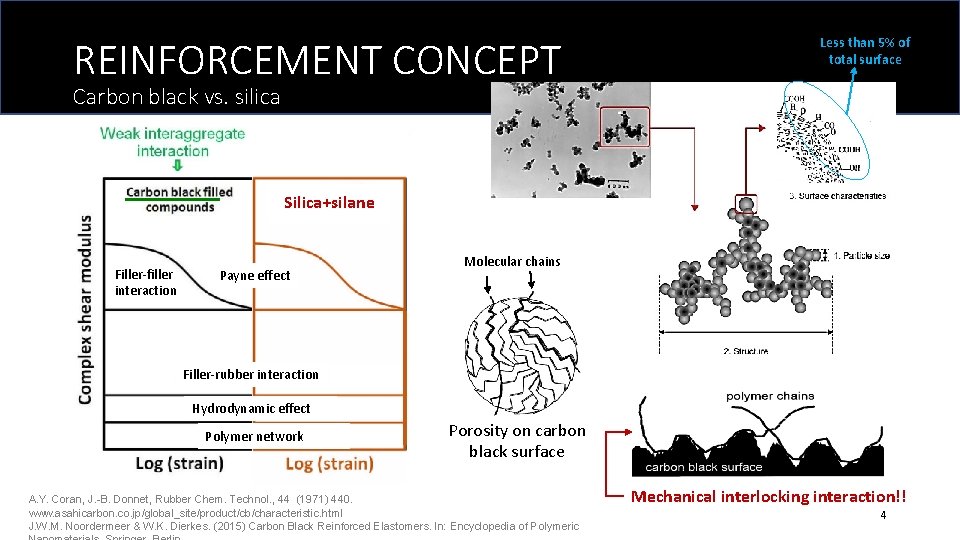
REINFORCEMENT CONCEPT Less than 5% of total surface Carbon black vs. silica Silica+silane (without silane) Filler-filler interaction Payne effect Molecular chains Filler-rubber interaction Hydrodynamic effect Polymer network Porosity on carbon black surface A. Y. Coran, J. -B. Donnet, Rubber Chem. Technol. , 44 (1971) 440. www. asahicarbon. co. jp/global_site/product/cb/characteristic. html J. W. M. Noordermeer & W. K. Dierkes. (2015) Carbon Black Reinforced Elastomers. In: Encyclopedia of Polymeric Mechanical interlocking interaction!! 4

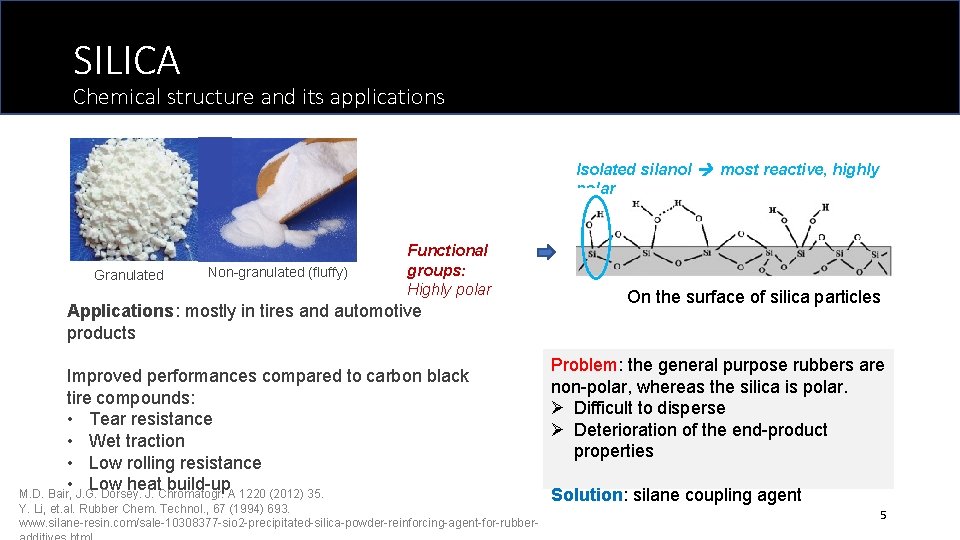
SILICA Chemical structure and its applications Isolated silanol most reactive, highly polar Granulated Non-granulated (fluffy) Functional groups: Highly polar Applications: mostly in tires and automotive products Improved performances compared to carbon black tire compounds: • Tear resistance • Wet traction • Low rolling resistance • Low heat build-up M. D. Bair, J. G. Dorsey. J. Chromatogr. A 1220 (2012) 35. Y. Li, et. al. Rubber Chem. Technol. , 67 (1994) 693. www. silane-resin. com/sale-10308377 -sio 2 -precipitated-silica-powder-reinforcing-agent-for-rubber- On the surface of silica particles Problem: the general purpose rubbers are non-polar, whereas the silica is polar. Ø Difficult to disperse Ø Deterioration of the end-product properties Solution: silane coupling agent 5

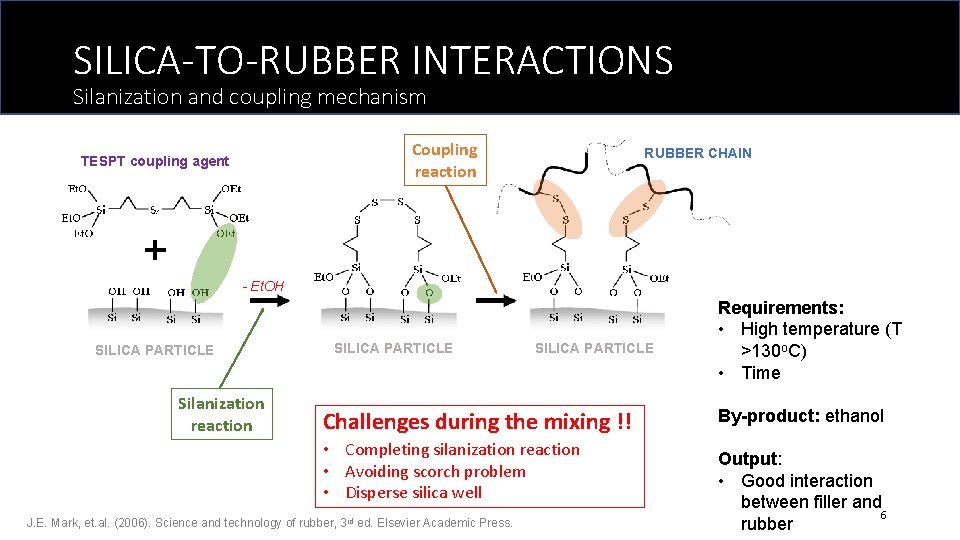
SILICA-TO-RUBBER INTERACTIONS Silanization and coupling mechanism Coupling reaction TESPT coupling agent RUBBER CHAIN - Et. OH SILICA PARTICLE Silanization reaction SILICA PARTICLE Challenges during the mixing !! • Completing silanization reaction • Avoiding scorch problem • Disperse silica well J. E. Mark, et. al. (2006). Science and technology of rubber, 3 rd ed. Elsevier Academic Press. Requirements: • High temperature (T >130 o. C) • Time By-product: ethanol Output: • Good interaction between filler and 6 rubber

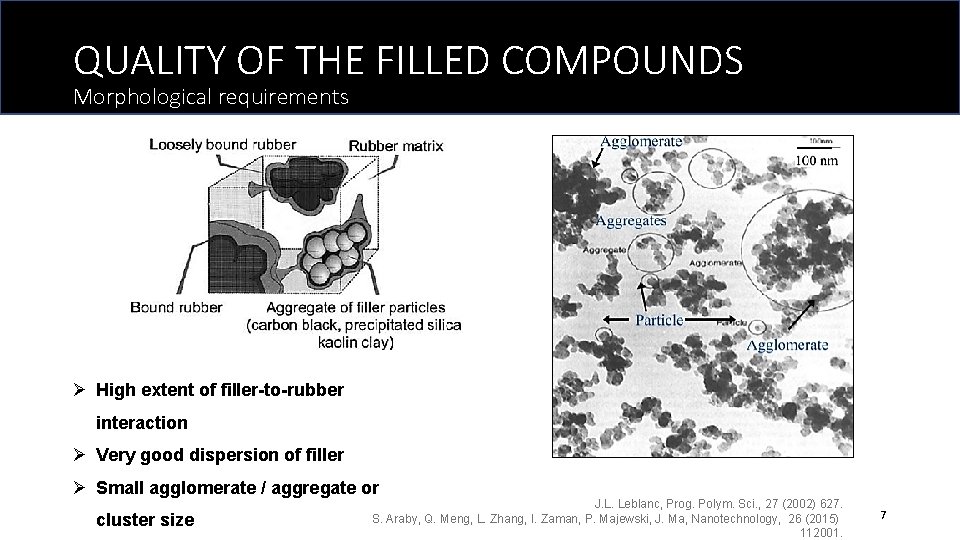
QUALITY OF THE FILLED COMPOUNDS Morphological requirements Ø High extent of filler-to-rubber interaction Ø Very good dispersion of filler Ø Small agglomerate / aggregate or cluster size J. L. Leblanc, Prog. Polym. Sci. , 27 (2002) 627. S. Araby, Q. Meng, L. Zhang, I. Zaman, P. Majewski, J. Ma, Nanotechnology, 26 (2015) 112001. 7

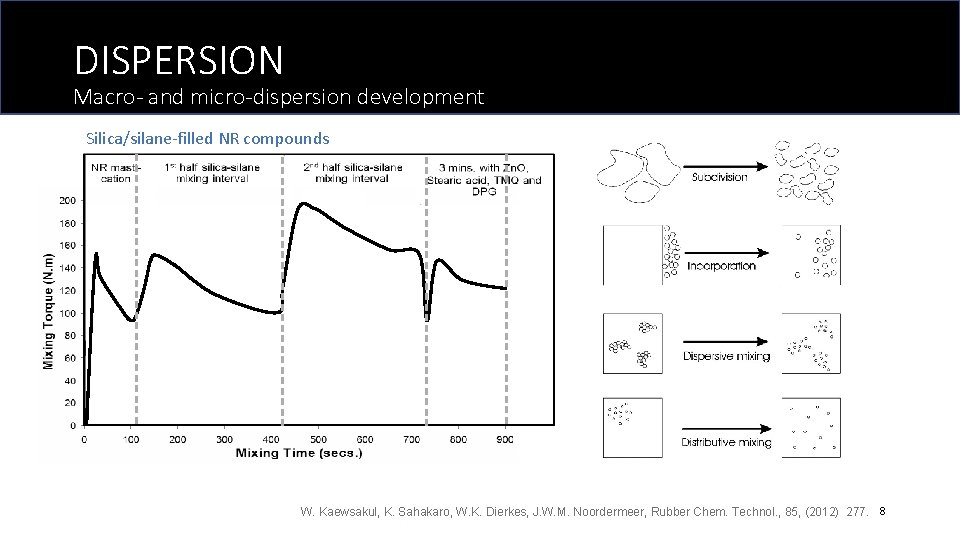
DISPERSION Macro- and micro-dispersion development Silica/silane-filled NR compounds W. Kaewsakul, K. Sahakaro, W. K. Dierkes, J. W. M. Noordermeer, Rubber Chem. Technol. , 85, (2012) 277. 8

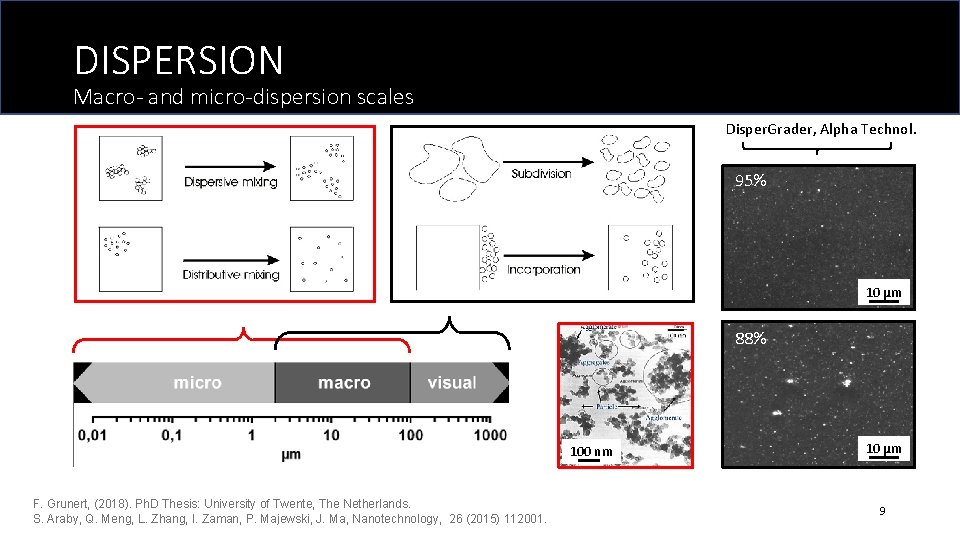
DISPERSION Macro- and micro-dispersion scales Disper. Grader, Alpha Technol. 95% 10 μm 88% 100 nm F. Grunert, (2018). Ph. D Thesis: University of Twente, The Netherlands. S. Araby, Q. Meng, L. Zhang, I. Zaman, P. Majewski, J. Ma, Nanotechnology, 26 (2015) 112001. 10 μm 9

2. RESEARCH QUESTIONS

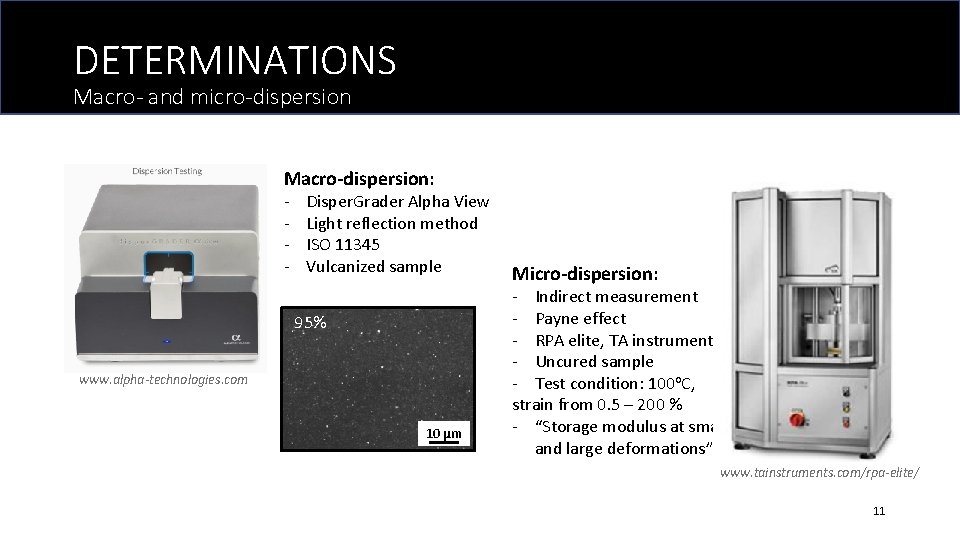
DETERMINATIONS Macro- and micro-dispersion Macro-dispersion: - Disper. Grader Alpha View Light reflection method ISO 11345 Vulcanized sample 95% www. alpha-technologies. com 10 μm Micro-dispersion: - Indirect measurement - Payne effect - RPA elite, TA instrument - Uncured sample - Test condition: 100 o. C, strain from 0. 5 – 200 % - “Storage modulus at small and large deformations” www. tainstruments. com/rpa-elite/ 11

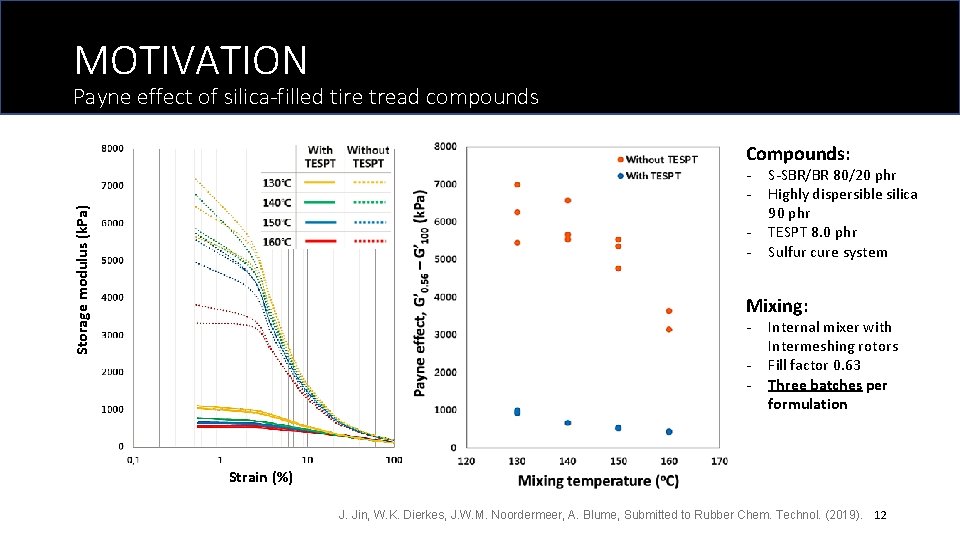
MOTIVATION Payne effect of silica-filled tire tread compounds Compounds: Storage modulus (k. Pa) - S-SBR/BR 80/20 phr Highly dispersible silica 90 phr TESPT 8. 0 phr Sulfur cure system Mixing: - Internal mixer with Intermeshing rotors Fill factor 0. 63 Three batches per formulation Strain (%) J. Jin, W. K. Dierkes, J. W. M. Noordermeer, A. Blume, Submitted to Rubber Chem. Technol. (2019). 12

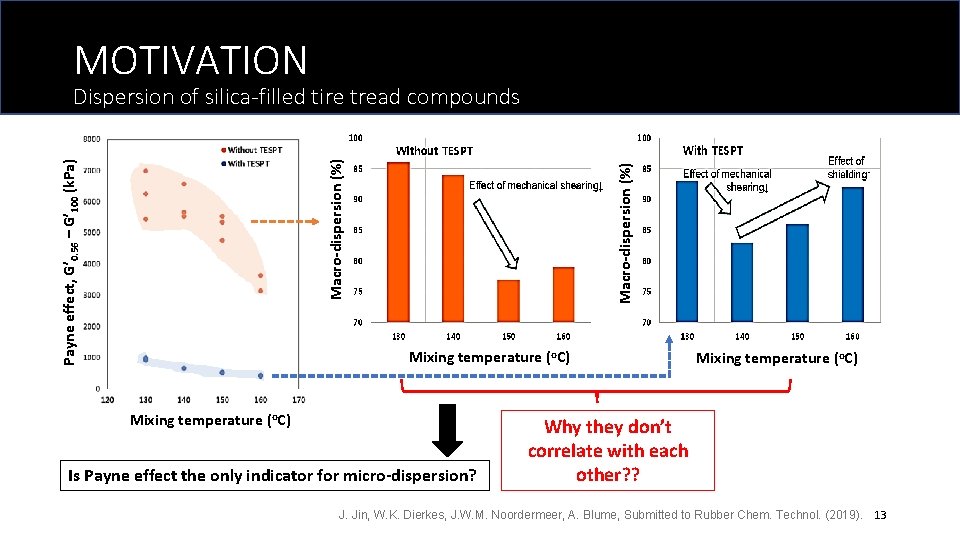
MOTIVATION Macro-dispersion (%) Payne effect, G’ 0. 56 – G’ 100 (k. Pa) Macro-dispersion (%) Dispersion of silica-filled tire tread compounds Mixing temperature (o. C) Is Payne effect the only indicator for micro-dispersion? Mixing temperature (o. C) Why they don’t correlate with each other? ? J. Jin, W. K. Dierkes, J. W. M. Noordermeer, A. Blume, Submitted to Rubber Chem. Technol. (2019). 13

OBJECTIVES Ø To verify the correlation between macro- and micro dispersion of silica-filled passenger car tire tread compounds Ø To gain a better understanding of the factors affecting the dispersion degree of silica-filled tire tread compounds ØThe compounds with different levels of silica dispersion were investigated: • Variation of mixing times • Variation of amounts of TESPT 14

3. EXPERIMENTS

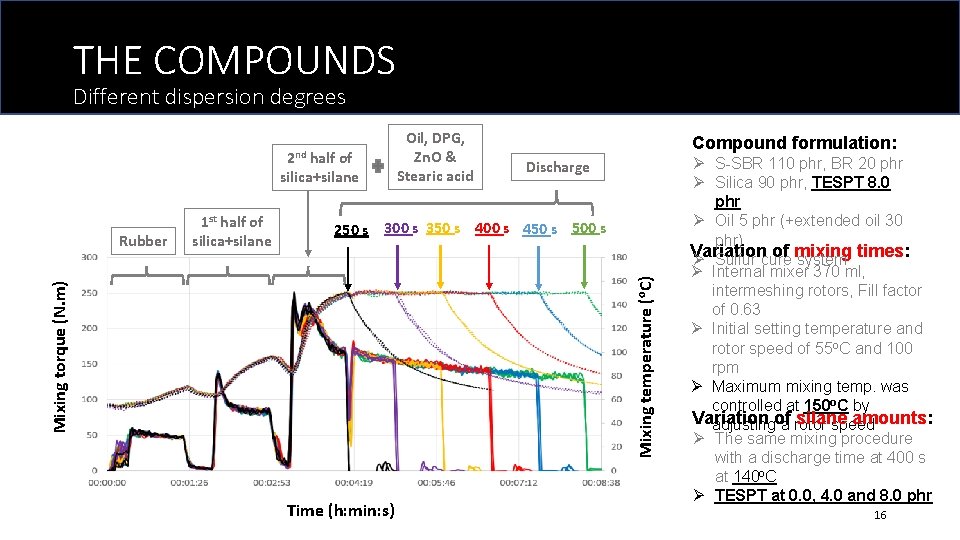
THE COMPOUNDS Different dispersion degrees 2 nd half of silica+silane Compound formulation: Discharge Mixing temperature (o. C) 250 s 300 s 350 s 400 s 450 s 500 s Mixing torque (N. m) Rubber 1 st half of silica+silane Oil, DPG, Zn. O & Stearic acid Time (h: min: s) Ø S-SBR 110 phr, BR 20 phr Ø Silica 90 phr, TESPT 8. 0 phr Ø Oil 5 phr (+extended oil 30 phr) Variation of mixing Ø Sulfur cure system times: Ø Internal mixer 370 ml, intermeshing rotors, Fill factor of 0. 63 Ø Initial setting temperature and rotor speed of 55 o. C and 100 rpm Ø Maximum mixing temp. was controlled at 150 o. C by Variation silane amounts: adjustingof a rotor speed Ø The same mixing procedure with a discharge time at 400 s at 140 o. C Ø TESPT at 0. 0, 4. 0 and 8. 0 phr 16

4. RESULTS

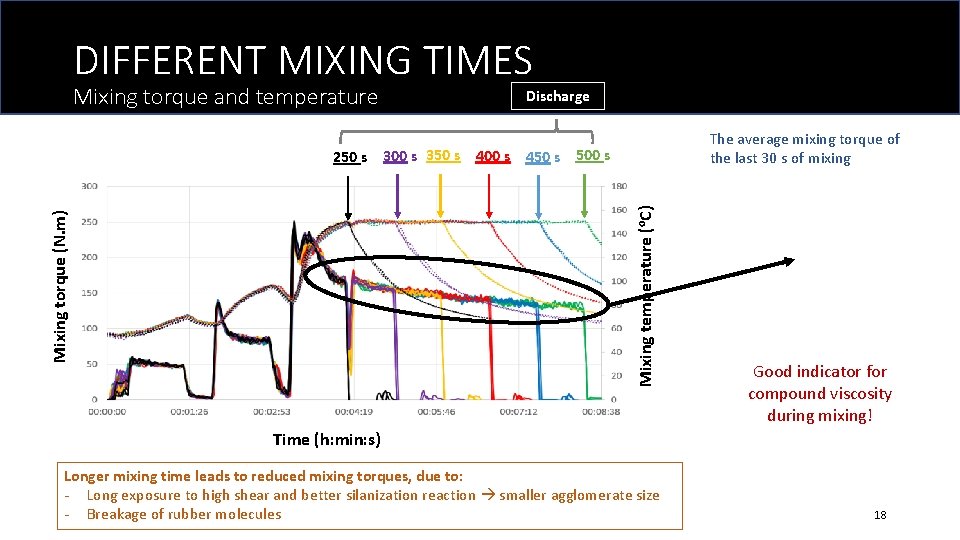
DIFFERENT MIXING TIMES Mixing torque and temperature Discharge The average mixing torque of the last 30 s of mixing Mixing torque (N. m) Mixing temperature (o. C) 250 s 300 s 350 s 400 s 450 s 500 s Good indicator for compound viscosity during mixing! Time (h: min: s) Longer mixing time leads to reduced mixing torques, due to: - Long exposure to high shear and better silanization reaction smaller agglomerate size - Breakage of rubber molecules 18

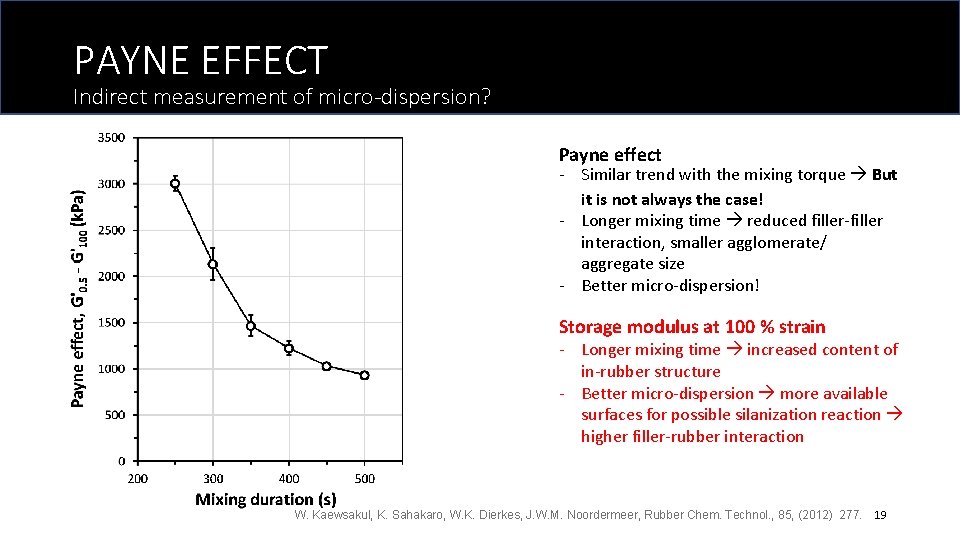
PAYNE EFFECT Indirect measurement of micro-dispersion? Payne effect - Similar trend with the mixing torque But it is not always the case! - Longer mixing time reduced filler-filler interaction, smaller agglomerate/ aggregate size - Better micro-dispersion! Storage modulus at 100 % strain - Longer mixing time increased content of in-rubber structure - Better micro-dispersion more available surfaces for possible silanization reaction higher filler-rubber interaction W. Kaewsakul, K. Sahakaro, W. K. Dierkes, J. W. M. Noordermeer, Rubber Chem. Technol. , 85, (2012) 277. 19

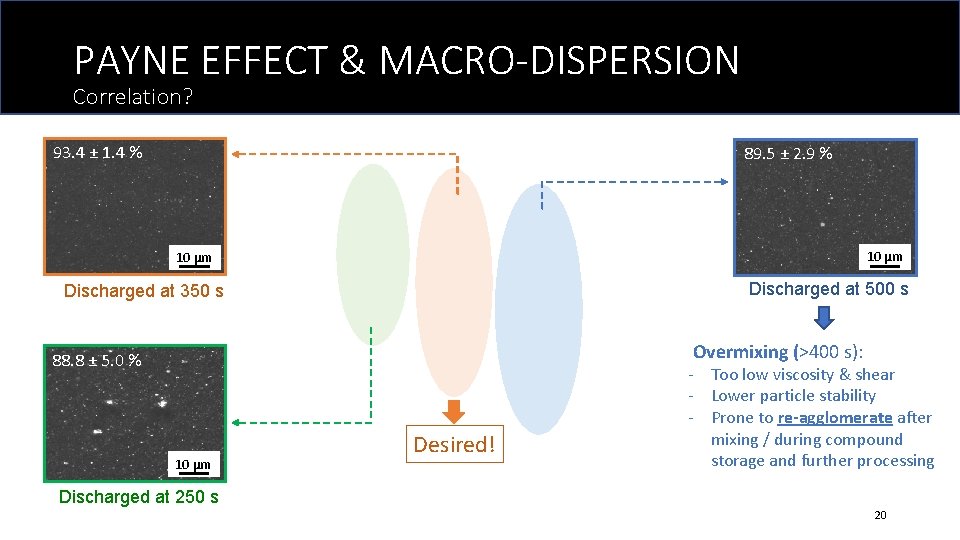
PAYNE EFFECT & MACRO-DISPERSION Correlation? 93. 4 ± 1. 4 % 89. 5 ± 2. 9 % 10 μm Discharged at 500 s Discharged at 350 s Overmixing (>400 s): 88. 8 ± 5. 0 % 10 μm Desired! - Too low viscosity & shear - Lower particle stability - Prone to re-agglomerate after mixing / during compound storage and further processing Discharged at 250 s 20

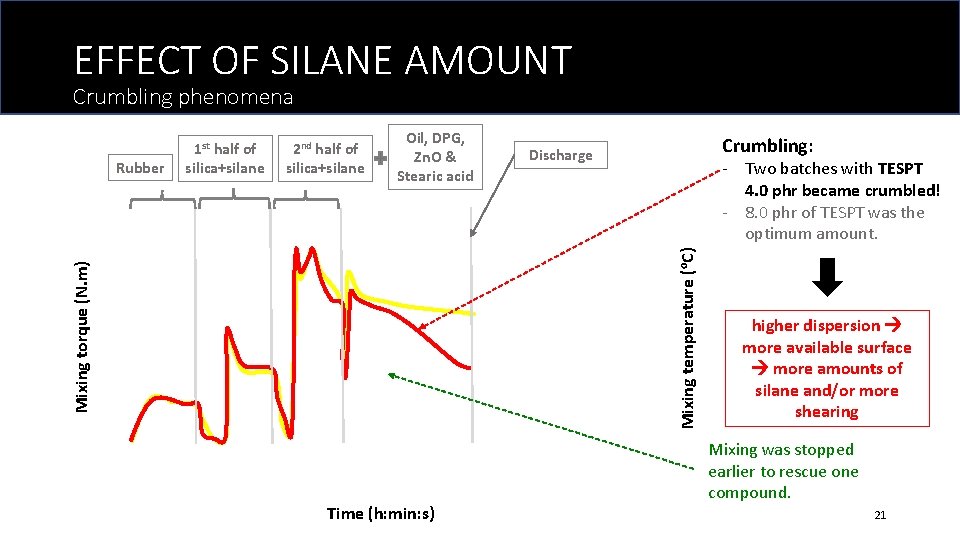
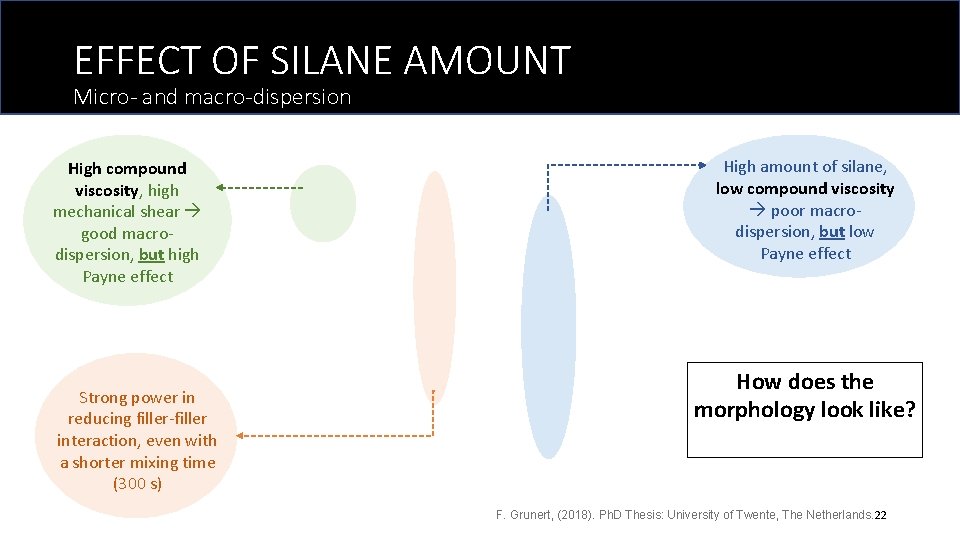
EFFECT OF SILANE AMOUNT Crumbling phenomena Oil, DPG, Zn. O & Stearic acid Crumbling: Discharge - Two batches with TESPT 4. 0 phr became crumbled! - 8. 0 phr of TESPT was the optimum amount. Mixing temperature (o. C) 2 nd half of silica+silane Mixing torque (N. m) Rubber 1 st half of silica+silane Time (h: min: s) higher dispersion more available surface more amounts of silane and/or more shearing Mixing was stopped earlier to rescue one compound. 21

EFFECT OF SILANE AMOUNT Micro- and macro-dispersion High compound viscosity, high mechanical shear good macrodispersion, but high Payne effect Strong power in reducing filler-filler interaction, even with a shorter mixing time (300 s) High amount of silane, low compound viscosity poor macrodispersion, but low Payne effect How does the morphology look like? F. Grunert, (2018). Ph. D Thesis: University of Twente, The Netherlands. 22

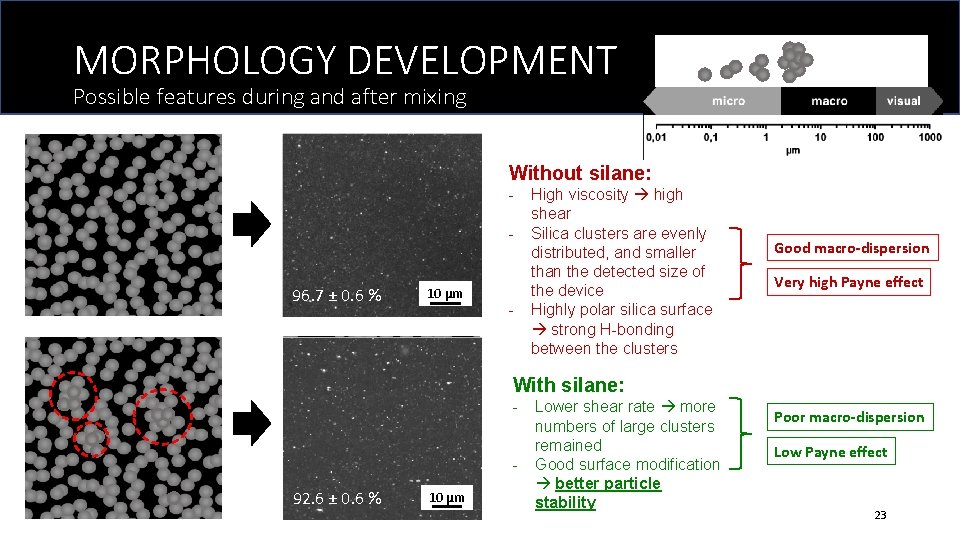
MORPHOLOGY DEVELOPMENT Possible features during and after mixing Without silane: - 96. 7 ± 0. 6 % 10 μm - High viscosity high shear Silica clusters are evenly distributed, and smaller than the detected size of the device Highly polar silica surface strong H-bonding between the clusters Good macro-dispersion Very high Payne effect With silane: - 92. 6 ± 0. 6 % 10 μm Lower shear rate more numbers of large clusters remained Good surface modification better particle stability Poor macro-dispersion Low Payne effect 23

6. CONCLUSIONS

CONCLUSIONS Correlation between macro- and micro-dispersion? Ø No correlation between them. Ø Mixing torque cannot be used to indicate the micro-dispersion of silica Factors affecting macro- and micro-dispersion of silica: Ø Mixing conditions § Mixing temperature § Mixing time Ø Rubber formulation § Silica type and amount § Silane type and amount § Viscosity modifiers 25

THANK YOU ! Wisut Kaewsakul w. kaewsakul@utwente. nl Elastomer Technology and Engineering Department of Mechanics of Solids, Surfaces and Systems Faculty of Engineering Technology University of Twente Enschede, The Netherlands